MT-ND6
| ND6 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | ND6, MTMT-NADH dehydrogenase, subunit 6 (complex I) | ||||||||||||||||||||||||
| External IDs | MGI: 102495 HomoloGene: 5022 GeneCards: ND6 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) |
|
| |||||||||||||||||||||||
| RefSeq (protein) |
| ||||||||||||||||||||||||
| Location (UCSC) | n/a | Chr M: 0.01 – 0.01 Mb | |||||||||||||||||||||||
| PubMed search | [2] | [3] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
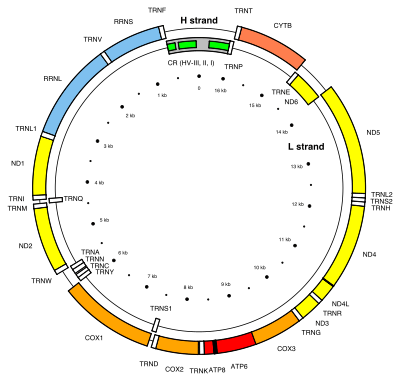
NADH-ubiquinone oxidoreductase chain 6 is a protein that in humans is encoded by the mitochondrial MT-ND6 gene.[4] The ND6 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain.[5] Variations in the MT-ND6 gene are associated with Leigh's syndrome, Leber's hereditary optic neuropathy (LHON) and dystonia.[6]
Structure
The MT-ND6 gene is located in human mitochondrial DNA from base pair 14,149 to 14,673.[4] MT-ND6 is the only protein-coding gene located on the L-strand of the human mitogenome.[7]
The encoded protein is 18 kDa and composed of 172 amino acids.[8][9] MT-ND6 is one of seven mitochondrially-encoded subunits of the enzyme NADH dehydrogenase (ubiquinone). Also known as Complex I, it is the largest of the respiratory complexes. The structure is L-shaped with a long, hydrophobic transmembrane domain and a hydrophilic domain for the peripheral arm that includes all the known redox centres and the NADH binding site. MT-ND6 and the rest of the mitochondrially encoded subunits are the most hydrophobic of the subunits of Complex I and form the core of the transmembrane region.[5]
Function
MT-ND6 is a subunit of the respiratory chain Complex I that is believed to belong to the minimal assembly of core proteins required to catalyze NADH dehydrogenation and electron transfer to ubiquinone (coenzyme Q10).[10] Initially, NADH binds to Complex I and transfers two electrons to the isoalloxazine ring of the flavin mononucleotide (FMN) prosthetic arm to form FMNH2. The electrons are transferred through a series of iron-sulfur (Fe-S) clusters in the prosthetic arm and finally to coenzyme Q10 (CoQ), which is reduced to ubiquinol (CoQH2). The flow of electrons changes the redox state of the protein, resulting in a conformational change and pK shift of the ionizable side chain, which pumps four hydrogen ions out of the mitochondrial matrix.[5]
Clinical significance
A T → C mutation at the 14484 base pair in the MT-ND6 gene has been identified in people with Leber's hereditary optic neuropathy (LHON). This common MT-ND6 mutation is responsible for about 14 percent of all cases of LHON, and it is the most common cause of this disorder among people of French Canadian descent. This mutation changes a single amino acid in the NADH dehydrogenase 6 protein at position 64, from methionine to valine. The T14484C mutation is associated with a good long-term prognosis; affected people with this genetic change have a 37 percent to 65 percent chance of some visual recovery. Researchers are investigating how mutations in the MT-ND6 gene lead to Leber's hereditary optic neuropathy. These genetic changes appear to prevent Complex I from interacting normally with ubiquinone, which may affect the generation of ATP and may also increase the production within mitochondria of potentially harmful molecules called reactive oxygen species (ROS). It remains unclear, however, why the effects of these mutations are often limited to the nerve that relays visual information from the eye to the brain (the optic nerve). Additional genetic and environmental factors probably contribute to the vision loss and other medical problems associated with Leber hereditary optic neuropathy.[6]
A G → A mutation at the 14459 base pair in the MT-ND6 gene also has been identified in a small number of people with Leigh's syndrome, a progressive brain disorder that typically appears in infancy or early childhood. Affected children may experience vomiting, seizures, delayed development, muscle weakness, and problems with movement. Heart disease, kidney problems, and difficulty breathing can also occur in people with this disorder. This MT-ND6 G14459A mutation replaces the amino acid alanine with the amino acid valine at protein position 72 in the NADH-ubiquinone oxidoreductase chain 6 protein. This genetic change also has been found in people with LHON and a movement disorder called dystonia, which involves involuntary muscle contractions, tremors, and other uncontrolled movements. This mutation appears to disrupt the normal assembly or activity of complex I in mitochondria. It is not known, however, how this MT-ND6 gene alteration is related to the specific features of Leigh syndrome, LHON, or dystonia. It also remains unclear why a single mutation can cause such varied signs and symptoms in different people.[6]
Interactions
MT-ND6 interacts with the NADH dehydrogenase [ubiquinone] iron-sulfur protein 3 (NDUFS3) and the ATP-dependent metalloprotease YME1L1.[4]
References
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000064368 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 "Entrez Gene: MT-ND6 NADH dehydrogenase subunit 6".
- 1 2 3 Donald Voet; Judith G. Voet; Charlotte W. Pratt (2013). "18". Fundamentals of biochemistry : life at the molecular level (4th ed.). Hoboken, NJ: Wiley. pp. 581–620. ISBN 9780470547847.
- 1 2 3 "MT-ND6". Genetics Home Reference. U.S. National Library of Medicine. Retrieved 23 March 2015.
- ↑ Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (April 1981). "Sequence and organization of the human mitochondrial genome". Nature. 290 (5806): 457–65. doi:10.1038/290457a0. PMID 7219534.
- ↑ Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (October 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- ↑ "NADH-ubiquinone oxidoreductase chain 6". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).
- ↑ "MT-ND6 - NADH-ubiquinone oxidoreductase chain 6 - Homo sapiens (Human)". UniProt.org: a hub for protein information. The UniProt Consortium.
Further reading
- Tarnopolsky M, Meaney B, Robinson B, Sheldon K, Boles RG (August 2013). "Severe infantile leigh syndrome associated with a rare mitochondrial ND6 mutation, m.14487T>C". American Journal of Medical Genetics. Part A. 161A (8): 2020–3. doi:10.1002/ajmg.a.36000. PMID 23813926.
- Torroni A, Achilli A, Macaulay V, Richards M, Bandelt HJ (June 2006). "Harvesting the fruit of the human mtDNA tree". Trends in Genetics. 22 (6): 339–45. doi:10.1016/j.tig.2006.04.001. PMID 16678300.
- Johns DR, Neufeld MJ, Park RD (September 1992). "An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy". Biochemical and Biophysical Research Communications. 187 (3): 1551–7. doi:10.1016/0006-291X(92)90479-5. PMID 1417830.
- Marzuki S, Noer AS, Lertrit P, Thyagarajan D, Kapsa R, Utthanaphol P, Byrne E (December 1991). "Normal variants of human mitochondrial DNA and translation products: the building of a reference data base". Human Genetics. 88 (2): 139–45. doi:10.1007/bf00206061. PMID 1757091.
- Moraes CT, Andreetta F, Bonilla E, Shanske S, DiMauro S, Schon EA (March 1991). "Replication-competent human mitochondrial DNA lacking the heavy-strand promoter region". Molecular and Cellular Biology. 11 (3): 1631–7. PMC 369459. PMID 1996112.
- Ozawa T, Tanaka M, Sugiyama S, Ino H, Ohno K, Hattori K, Ohbayashi T, Ito T, Deguchi H, Kawamura K (May 1991). "Patients with idiopathic cardiomyopathy belong to the same mitochondrial DNA gene family of Parkinson's disease and mitochondrial encephalomyopathy". Biochemical and Biophysical Research Communications. 177 (1): 518–25. doi:10.1016/0006-291X(91)92014-B. PMID 2043137.
- Chomyn A, Cleeter MW, Ragan CI, Riley M, Doolittle RF, Attardi G (October 1986). "URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit". Science. 234 (4776): 614–8. doi:10.1126/science.3764430. PMID 3764430.
- Chomyn A, Mariottini P, Cleeter MW, Ragan CI, Matsuno-Yagi A, Hatefi Y, Doolittle RF, Attardi G (1985). "Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase". Nature. 314 (6012): 592–7. doi:10.1038/314592a0. PMID 3921850.
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (April 1981). "Sequence and organization of the human mitochondrial genome". Nature. 290 (5806): 457–65. doi:10.1038/290457a0. PMID 7219534.
- Montoya J, Ojala D, Attardi G (April 1981). "Distinctive features of the 5'-terminal sequences of the human mitochondrial mRNAs". Nature. 290 (5806): 465–70. doi:10.1038/290465a0. PMID 7219535.
- Horai S, Hayasaka K, Kondo R, Tsugane K, Takahata N (January 1995). "Recent African origin of modern humans revealed by complete sequences of hominoid mitochondrial DNAs". Proceedings of the National Academy of Sciences of the United States of America. 92 (2): 532–6. doi:10.1073/pnas.92.2.532. PMC 42775. PMID 7530363.
- Jun AS, Brown MD, Wallace DC (June 1994). "A mitochondrial DNA mutation at nucleotide pair 14459 of the NADH dehydrogenase subunit 6 gene associated with maternally inherited Leber hereditary optic neuropathy and dystonia". Proceedings of the National Academy of Sciences of the United States of America. 91 (13): 6206–10. doi:10.1073/pnas.91.13.6206. PMC 44167. PMID 8016139.
- De Vries DD, Went LN, Bruyn GW, Scholte HR, Hofstra RM, Bolhuis PA, van Oost BA (April 1996). "Genetic and biochemical impairment of mitochondrial complex I activity in a family with Leber hereditary optic neuropathy and hereditary spastic dystonia". American Journal of Human Genetics. 58 (4): 703–11. PMC 1914692. PMID 8644732.
- Leo-Kottler B, Christ-Adler M, Baumann B, Zrenner E, Wissinger B (July 1996). "Leber's hereditary optic neuropathy: clinical and molecular genetic results obtained in a family with a new point mutation at nucleotide position 14498 in the ND 6 gene". German Journal of Ophthalmology. 5 (4): 233–40. PMID 8854108.
- Sudoyo H, Sitepu M, Malik S, Poesponegoro HD, Marzuki S (1999). "Leber's hereditary optic neuropathy in Indonesia: two families with the mtDNA 11778G>A and 14484T>C mutations". Human Mutation. Suppl 1: S271–4. doi:10.1002/humu.1380110186. PMID 9452107.
- Besch D, Leo-Kottler B, Zrenner E, Wissinger B (September 1999). "Leber's hereditary optic neuropathy: clinical and molecular genetic findings in a patient with a new mutation in the ND6 gene". Graefe's Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. 237 (9): 745–52. doi:10.1007/s004170050307. PMID 10447650.
- Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N (October 1999). "Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA". Nature Genetics. 23 (2): 147. doi:10.1038/13779. PMID 10508508.
- Ingman M, Kaessmann H, Pääbo S, Gyllensten U (December 2000). "Mitochondrial genome variation and the origin of modern humans". Nature. 408 (6813): 708–13. doi:10.1038/35047064. PMID 11130070.
- Chinnery PF, Brown DT, Andrews RM, Singh-Kler R, Riordan-Eva P, Lindley J, Applegarth DA, Turnbull DM, Howell N (January 2001). "The mitochondrial ND6 gene is a hot spot for mutations that cause Leber's hereditary optic neuropathy". Brain. 124 (Pt 1): 209–18. doi:10.1093/brain/124.1.209. PMID 11133798.
- Finnilä S, Lehtonen MS, Majamaa K (June 2001). "Phylogenetic network for European mtDNA". American Journal of Human Genetics. 68 (6): 1475–84. doi:10.1086/320591. PMC 1226134. PMID 11349229.
- Maca-Meyer N, González AM, Larruga JM, Flores C, Cabrera VM (2003). "Major genomic mitochondrial lineages delineate early human expansions". BMC Genetics. 2: 13. doi:10.1186/1471-2156-2-13. PMC 55343. PMID 11553319.
External links
- Mass spectrometry characterization of MT-ND6 at COPaKB
- GeneReviews/NCBI/NIH/UW entry on Mitochondrial DNA-Associated Leigh Syndrome and NARP
This article incorporates text from the United States National Library of Medicine, which is in the public domain.