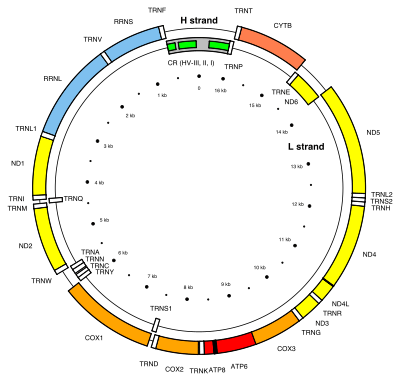
MT-ND1
NADH-ubiquinone oxidoreductase chain 1 is a protein that in humans is encoded by the mitochondrial gene MT-ND1.[4] The ND1 protein is a subunit of NADH dehydrogenase, which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain.[5] Variants of the MT-ND1 gene are associated with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS), Leigh's syndrome (LS), Leber's hereditary optic neuropathy (LHON) and increases in adult BMI.[6][7][8]
Structure
MT-ND1 is located in mitochondrial DNA from base pair 3,307 to 4,262.[4] The MT-ND1 gene produces a 36 kDa protein composed of 318 amino acids.[9][10] MT-ND1 is one of seven mitochondrially-encoded subunits of the enzyme NADH dehydrogenase (ubiquinone). Also known as Complex I, it is the largest of the respiratory complexes. The structure is L-shaped with a long, hydrophobic transmembrane domain and a hydrophilic domain for the peripheral arm that includes all the known redox centres and the NADH binding site. MT-ND1 and the rest of the mitochondrially encoded subunits are the most hydrophobic of the subunits of Complex I and form the core of the transmembrane region.[5]
Function
MT-ND1 is a subunit of the respiratory chain Complex I that is believed to belong to the minimal assembly of core proteins required to catalyze NADH dehydrogenation and electron transfer to ubiquinone (coenzyme Q10).[11] Initially, NADH binds to Complex I and transfers two electrons to the isoalloxazine ring of the flavin mononucleotide (FMN) prosthetic arm to form FMNH2. The electrons are transferred through a series of iron-sulfur (Fe-S) clusters in the prosthetic arm and finally to coenzyme Q10 (CoQ), which is reduced to ubiquinol (CoQH2). The flow of electrons changes the redox state of the protein, resulting in a conformational change and pK shift of the ionizable side chain, which pumps four hydrogen ions out of the mitochondrial matrix.[5]
Clinical significance
Pathogenic variants of the mitochondrial gene MT-ND1 are known to cause mtDNA-associated Leigh syndrome, as are variants of MT-ATP6, MT-TL1, MT-TK, MT-TW, MT-TV, MT-ND2, MT-ND3, MT-ND4, MT-ND5, MT-ND6 and MT-CO3. Abnormalities in mitochondrial energy generation result in neurodegenerative disorders like Leigh syndrome, which is characterized by an onset of symptoms between 12 months and three years of age. The symptoms frequently present themselves following a viral infection and include movement disorders and peripheral neuropathy, as well as hypotonia, spasticity and cerebellar ataxia. Roughly half of affected individuals die of respiratory or cardiac failure by the age of three. Leigh syndrome is a maternally inherited disorder and its diagnosis is established through genetic testing of the aforementioned mitochondrial genes, including MT-ND1.[6] The m.4171C>A/MT-ND1 mutation also leads to a Leigh-like phenotype as well as bilateral brainstem lesions affecting the vestibular nuclei, resulting in vision loss, vomiting and vertigo.[7] These complex I genes have been associated with a variety of neurodegenerative disorders, including Leber's hereditary optic neuropathy (LHON), mitochondrial encephalomyopathy with stroke-like episodes (MELAS), overlap between LHON and MELAS,[12][13] and the previously mentioned Leigh syndrome.
Mitochondrial dysfunction resulting from variants of MT-ND1, MT-ND2 and MT-ND4L have been linked to BMI in adults and implicated in metabolic disorders including obesity, diabetes and hypertension.[8]
References
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000064341 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 "Entrez Gene: MT-ND1 NADH dehydrogenase subunit 1".
- 1 2 3 Voet DJ, Voet JG, Pratt CW (2013). "Chapter 18, Mitochondrial ATP synthesis". Fundamentals of Biochemistry (4th ed.). Hoboken, NJ: Wiley. pp. 581–620. ISBN 978-0-47054784-7.
- 1 2 Thorburn DR, Rahman S (1993–2015). "Mitochondrial DNA-Associated Leigh Syndrome and NARP". In Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, Bird TD, Dolan CR, Fong CT, Smith RJ, Stephens K. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle.
- 1 2 La Morgia C, Caporali L, Gandini F, Olivieri A, Toni F, Nassetti S, Brunetto D, Stipa C, Scaduto C, Parmeggiani A, Tonon C, Lodi R, Torroni A, Carelli V (May 2014). "Association of the mtDNA m.4171C>A/MT-ND1 mutation with both optic neuropathy and bilateral brainstem lesions". BMC Neurology. 14: 116. doi:10.1186/1471-2377-14-116. PMC 4047257. PMID 24884847.
- 1 2 Flaquer A, Baumbach C, Kriebel J, Meitinger T, Peters A, Waldenberger M, Grallert H, Strauch K (2014). "Mitochondrial genetic variants identified to be associated with BMI in adults". PLOS One. 9 (8): e105116. doi:10.1371/journal.pone.0105116. PMC 4143221. PMID 25153900.
- ↑ Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (October 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- ↑ "NADH-ubiquinone oxidoreductase chain 1". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).
- ↑ "MT-ND1 - NADH-ubiquinone oxidoreductase chain 1 - Homo sapiens (Human)". UniProt.org: a hub for protein information. The UniProt Consortium.
- ↑ Spruijt L, Smeets HJ, Hendrickx A, Bettink-Remeijer MW, Maat-Kievit A, Schoonderwoerd KC, Sluiter W, de Coo IF, Hintzen RQ (June 2007). "A MELAS-associated ND1 mutation causing leber hereditary optic neuropathy and spastic dystonia". Archives of Neurology. 64 (6): 890–3. doi:10.1001/archneur.64.6.890. PMID 17562939.
- ↑ Blakely EL, de Silva R, King A, Schwarzer V, Harrower T, Dawidek G, Turnbull DM, Taylor RW (May 2005). "LHON/MELAS overlap syndrome associated with a mitochondrial MTND1 gene mutation". European Journal of Human Genetics. 13 (5): 623–7. doi:10.1038/sj.ejhg.5201363. PMID 15657614.
Further reading
- Torroni A, Achilli A, Macaulay V, Richards M, Bandelt HJ (June 2006). "Harvesting the fruit of the human mtDNA tree". Trends in Genetics. 22 (6): 339–45. doi:10.1016/j.tig.2006.04.001. PMID 16678300.
- Bodenteich A, Mitchell LG, Polymeropoulos MH, Merril CR (May 1992). "Dinucleotide repeat in the human mitochondrial D-loop". Human Molecular Genetics. 1 (2): 140. doi:10.1093/hmg/1.2.140-a. PMID 1301157.
- Lu X, Walker T, MacManus JP, Seligy VL (July 1992). "Differentiation of HT-29 human colonic adenocarcinoma cells correlates with increased expression of mitochondrial RNA: effects of trehalose on cell growth and maturation". Cancer Research. 52 (13): 3718–25. PMID 1377597.
- Johns DR, Neufeld MJ, Park RD (September 1992). "An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy". Biochemical and Biophysical Research Communications. 187 (3): 1551–7. doi:10.1016/0006-291X(92)90479-5. PMID 1417830.
- Huoponen K, Vilkki J, Aula P, Nikoskelainen EK, Savontaus ML (June 1991). "A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy". American Journal of Human Genetics. 48 (6): 1147–53. PMC 1683111. PMID 1674640.
- Marzuki S, Noer AS, Lertrit P, Thyagarajan D, Kapsa R, Utthanaphol P, Byrne E (December 1991). "Normal variants of human mitochondrial DNA and translation products: the building of a reference data base". Human Genetics. 88 (2): 139–45. doi:10.1007/bf00206061. PMID 1757091.
- Johns DR, Berman J (February 1991). "Alternative, simultaneous complex I mitochondrial DNA mutations in Leber's hereditary optic neuropathy". Biochemical and Biophysical Research Communications. 174 (3): 1324–30. doi:10.1016/0006-291X(91)91567-V. PMID 1900003.
- Howell N, Bindoff LA, McCullough DA, Kubacka I, Poulton J, Mackey D, Taylor L, Turnbull DM (November 1991). "Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees". American Journal of Human Genetics. 49 (5): 939–50. PMC 1683233. PMID 1928099.
- Majander A, Huoponen K, Savontaus ML, Nikoskelainen E, Wikström M (November 1991). "Electron transfer properties of NADH:ubiquinone reductase in the ND1/3460 and the ND4/11778 mutations of the Leber hereditary optic neuroretinopathy (LHON)". FEBS Letters. 292 (1–2): 289–92. doi:10.1016/0014-5793(91)80886-8. PMID 1959619.
- Moraes CT, Andreetta F, Bonilla E, Shanske S, DiMauro S, Schon EA (March 1991). "Replication-competent human mitochondrial DNA lacking the heavy-strand promoter region". Molecular and Cellular Biology. 11 (3): 1631–7. PMC 369459. PMID 1996112.
- Howell N, Kubacka I, Xu M, McCullough DA (May 1991). "Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation". American Journal of Human Genetics. 48 (5): 935–42. PMC 1683051. PMID 2018041.
- Attardi G, Chomyn A, Doolittle RF, Mariottini P, Ragan CI (1987). "Seven unidentified reading frames of human mitochondrial DNA encode subunits of the respiratory chain NADH dehydrogenase". Cold Spring Harbor Symposia on Quantitative Biology. 51 Pt 1 (1): 103–14. doi:10.1101/sqb.1986.051.01.013. PMID 3472707.
- Chomyn A, Cleeter MW, Ragan CI, Riley M, Doolittle RF, Attardi G (October 1986). "URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit". Science. 234 (4776): 614–8. doi:10.1126/science.3764430. PMID 3764430.
- Chomyn A, Mariottini P, Cleeter MW, Ragan CI, Matsuno-Yagi A, Hatefi Y, Doolittle RF, Attardi G (1985). "Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase". Nature. 314 (6012): 592–7. doi:10.1038/314592a0. PMID 3921850.
- Sanger F, Coulson AR, Barrell BG, Smith AJ, Roe BA (October 1980). "Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing". Journal of Molecular Biology. 143 (2): 161–78. doi:10.1016/0022-2836(80)90196-5. PMID 6260957.
- Pagani S, Galante YM (January 1983). "Interaction of rhodanese with mitochondrial NADH dehydrogenase". Biochimica et Biophysica Acta. 742 (2): 278–84. doi:10.1016/0167-4838(83)90312-6. PMID 6402020.
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (April 1981). "Sequence and organization of the human mitochondrial genome". Nature. 290 (5806): 457–65. doi:10.1038/290457a0. PMID 7219534.
- Montoya J, Ojala D, Attardi G (April 1981). "Distinctive features of the 5'-terminal sequences of the human mitochondrial mRNAs". Nature. 290 (5806): 465–70. doi:10.1038/290465a0. PMID 7219535.
- Horai S, Hayasaka K, Kondo R, Tsugane K, Takahata N (January 1995). "Recent African origin of modern humans revealed by complete sequences of hominoid mitochondrial DNAs". Proceedings of the National Academy of Sciences of the United States of America. 92 (2): 532–6. doi:10.1073/pnas.92.2.532. PMC 42775. PMID 7530363.
- Nakagawa Y, Ikegami H, Yamato E, Takekawa K, Fujisawa T, Hamada Y, Ueda H, Uchigata Y, Miki T, Kumahara Y (April 1995). "A new mitochondrial DNA mutation associated with non-insulin-dependent diabetes mellitus". Biochemical and Biophysical Research Communications. 209 (2): 664–8. doi:10.1006/bbrc.1995.1550. PMID 7733935.
- Delmiro A, Rivera H, García-Silva MT, García-Consuegra I, Martín-Hernández E, Quijada-Fraile P, de Las Heras RS, Moreno-Izquierdo A, Martín MÁ, Arenas J, Martínez-Azorín F (December 2013). "Whole-exome sequencing identifies a variant of the mitochondrial MT-ND1 gene associated with epileptic encephalopathy: west syndrome evolving to Lennox-Gastaut syndrome". Human Mutation. 34 (12): 1623–7. doi:10.1002/humu.22445. PMID 24105702.
- Musumeci O, Andreu AL, Shanske S, Bresolin N, Comi GP, Rothstein R, Schon EA, DiMauro S (June 2000). "Intragenic inversion of mtDNA: a new type of pathogenic mutation in a patient with mitochondrial myopathy". American Journal of Human Genetics. 66 (6): 1900–4. doi:10.1086/302927. PMID 10775530.