Lymphedema
| Lymphedema | |
|---|---|
| Synonyms | lymphoedema, lymphatic obstruction |
 | |
| Lymphedema on a 67-year-old female | |
| Specialty | General surgery |
Lymphedema, also known as lymphoedema and lymphatic edema, is a condition of localized fluid retention and tissue swelling caused by a compromised lymphatic system, which normally returns interstitial fluid to the bloodstream. The condition is most frequently a complication of cancer treatment or parasitic infections, but it can also be seen in a number of genetic disorders. Though incurable and progressive, a number of treatments can ameliorate symptoms. Tissues with lymphedema are at high risk of infection.
Signs and symptoms
The most common manifestation of lymphedema is soft tissue swelling, edema. As the disorder progresses, worsening edema and skin changes including discoloration, verrucous (wart-like) hyperplasia, hyperkeratosis, papillomatosis, dermal thickening and ulcers may be seen. Additionally, there is increased risk of infection of the superficial soft tissues, known as cellulitis.
Lymphedema should not be confused with edema arising from venous insufficiency, which is caused by compromise of the venous drainage rather than lymphatic drainage. However, untreated venous insufficiency can progress into a combined venous/lymphatic disorder.
Complications
When the lymphatic impairment becomes so great that the lymph fluid exceeds the lymphatic system's ability to transport it, an abnormal amount of protein-rich fluid collects in the tissues. Left untreated, this stagnant, protein-rich fluid causes tissue channels to increase in size and number, reducing oxygen availability. This interferes with wound healing and provides a rich culture medium for bacterial growth that can result in infections: cellulitis, lymphangitis, lymphadenitis and in severe cases, skin ulcers.[1] It is vital for lymphedema patients to be aware of the symptoms of infection and to seek immediate treatment, since recurrent infections or cellulitis, in addition to their inherent danger, further damage the lymphatic system and set up a vicious circle.
In rare cases, lymphedema can lead to a form of cancer called lymphangiosarcoma, although the mechanism of carcinogenesis is not understood. Lymphedema-associated lymphangiosarcoma is called Stewart-Treves syndrome.[1] Lymphangiosarcoma most frequently occurs in cases of long-standing lymphedema. The incidence of angiosarcoma is estimated to be 0.45% in patients living 5 years after radical mastectomy.[2][3] Lymphedema is also associated with a low grade form of cancer called retiform hemangioendothelioma (a low grade angiosarcoma).[4]
Since lymphedema is disfiguring, causing difficulties in daily living and can lead to lifestyle becoming severely limited, it may also result in psychological distress.
Causes
Lymphedema may be inherited (primary) or caused by injury to the lymphatic vessels (secondary). It is most frequently seen after lymph node dissection, surgery and/or radiation therapy, in which damage to the lymphatic system is caused during the treatment of cancer, most notably breast cancer. In many patients with cancer, this condition does not develop until months or even years after therapy has concluded. Lymphedema may also be associated with accidents or certain diseases or problems that may inhibit the lymphatic system from functioning properly.[1] In tropical areas of the world, a common cause of secondary lymphedema is filariasis, a parasitic infection. It can also be caused by damage to the lymphatic system from infections such as cellulitis.
Primary lymphedema may be inherited or arise sporadically. Multiple syndromes are associated with primary lymphedema, including Turner syndrome, Milroy's disease, and Klippel-Trenaunay-Weber syndrome. It generally thought to occur as a result of absent or malformed lymph nodes and/or lymphatic channels. Lymphedema may be present at birth, develop at the onset of puberty (praecox), or not become apparent for many years into adulthood (tarda). In men, lower-limb primary lymphedema is most common, occurring in one or both legs. Some cases of lymphedema may be associated with other vascular abnormalities.[1]
Secondary lymphedema affects both men and women. In women, it is most prevalent in the upper limbs after breast cancer surgery, in particular after axillary lymph node dissection,[5] occurring in the arm on the side of the body in which the surgery is performed. Breast and trunk lymphedema can also occur but go unrecognised as there is swelling in the area after surgery, and its symptoms (peau d'orange and/or an inverted nipple) can be confused with post surgery fat necrosis.[6] In Western countries, secondary lymphedema is most commonly due to cancer treatment.[7] Between 38 and 89% of breast cancer patients suffer from lymphedema due to axillary lymph node dissection and/or radiation.[7][8][9] Unilateral lymphedema occurs in up to 41% of patients after gynecologic cancer.[7][10] For men, a 5-66% incidence of lymphedema has been reported in patients treated with incidence depending on whether staging or radical removal of lymph glands was done in addition to radiotherapy.[7][11][12]
Head and neck lymphedema can be caused by surgery or radiation therapy for tongue or throat cancer. It may also occur in the lower limbs or groin after surgery for colon, ovarian or uterine cancer, in which removal of lymph nodes or radiation therapy is required. Surgery or treatment for prostate, colon and testicular cancers may result in secondary lymphedema, particularly when lymph nodes have been removed or damaged.
The onset of secondary lymphedema in patients who have had cancer surgery has also been linked to aircraft flight (likely due to decreased cabin pressure or relative immobility). For cancer survivors, therefore, wearing a prescribed and properly fitted compression garment may help decrease swelling during air travel.
Some cases of lower-limb lymphedema have been associated with the use of tamoxifen, due to the blood clots and deep vein thrombosis (DVT) that can be associated with this medication. Resolution of the blood clots or DVT is needed before lymphedema treatment can be initiated.
Congenital lymphedema
Congenital lymphedema is swelling that results from abnormalities in the lymphatic system that are present from birth. Swelling may be present in a single affected limb, several limbs, genitalia, or the face. It is sometimes diagnosed prenatally by a nuchal scan or post-natally by lymphoscintigraphy. One hereditary form of congenital lymphedema is called Milroy's disease and is caused by mutations in the VEGFR3 gene.[1][13] Congenital lymphedema is frequently syndromic and is associated with Turner syndrome, lymphedema–distichiasis syndrome, yellow nail syndrome, and Klippel–Trénaunay–Weber syndrome.[14]
One defined genetic cause for congenital lymphedema is GATA2 deficiency. This deficiency is a grouping of several disorders caused by common defect, viz., familial or sporadic inactivating mutations in one of the two parental GATA2 genes. These autosomal dominant mutations cause a reduction, i.e. a haploinsufficiency, in the cellular levels of the gene's product, GATA2. The GATA2 protein is a transcription factor critical for the embryonic development, maintenance, and functionality of blood-forming, lympathic-forming, and other tissue-forming stem cells. In consequence of these mutations, cellular levels of GATA2 are deficient and individuals develop over time hematological, immunological, lymphatic, and/or other disorders. GATA2 deficiency-induced defects in the lymphatic vessels and valves underlies the development of lymphedema with is primarily located in the lower extremities but may also occur in other places such as the face or testes (i.e. hydrocele). This form of the deficiency, when coupled with sensorineural hearing loss which may also be due to faulty development of the lymphatic system, is sometimes termed the Emberger syndrome.[15][16]
Primary lymphedema has a quoted incidence of approximately 1-3 births out of every 10,000 births, with a particular female preponderance to male ratio of 3.5:1 In North America, the incidence of primary lymphedema is approximately 1.15 births out of every 100,000 births Compared to secondary lymphedema, primary lymphedema is relatively rare.[17]
Physiology
Lymph is formed from the fluid that filters out of the blood circulation and contains proteins, cellular debris, bacteria, etc. The collection of this fluid is carried out by the initial lymph collectors that are blind-ended epithelial-lined vessels with fenestrated openings that allow fluids and particles as large as cells to enter. Once inside the lumen of the lymphatic vessels, the fluid is guided along increasingly larger vessels, first with rudimentary valves to prevent backflow, which later develop into complete valves similar to the venous valve. Once the lymph enters the fully valved lymphatic vessels, it is pumped by a rhythmic peristaltic-like action by smooth muscle cells within the lymphatic vessel walls. This peristaltic action is the primary driving force, moving lymph within its vessel walls. The regulation of the frequency and power of contraction is regulated by the sympathetic nervous system. Lymph movement can be influenced by the pressure of nearby muscle contraction, arterial pulse pressure and the vacuum created in the chest cavity during respiration, but these passive forces contribute only a minor percentage of lymph transport. The fluids collected are pumped into continually larger vessels and through lymph nodes, which remove debris and police the fluid for dangerous microbes. The lymph ends its journey in the thoracic duct or right lymphatic duct, which drain into the blood circulation.
Diagnosis
Accurate diagnosis and staging are fundamental to the management of lymphedema patients.[18] A swollen limb can result from different conditions that require different treatments. Diagnosis of lymphedema is currently based on history, physical exam, limb measurements, and imaging studies such as lymphoscintigraphy and indocyanine green lymphography. However, the ideal method for lymphedema staging to guide the most appropriate treatment is controversial because of several different proposed protocols.[19][20] Lymphedema can occur in both the upper and lower extremities, and in some cases, the head and neck. Assessment of the extremities first begins with a visual inspection. Color, presence of hair, visible veins, size and any sores or ulcerations are noted. Lack of hair may indicate an arterial circulation problem.[21] Given swelling, the extremities' circumference is measured for reference as time continues. In early stages of lymphedema, elevating the limb may reduce or eliminate the swelling. Palpation of the wrist or ankle can determine the degree of swelling; assessment includes a check of the pulses. The axillary or inguinal nodes may be enlarged due to the swelling. Enlargement of the nodes lasting more than three weeks may indicate infection or other illnesses such as sequela from breast cancer surgery requiring further medical attention.[21]
Diagnosis or early detection of lymphedema is difficult. The first signs may be subjective observations such as a feeling of heaviness in the affected extremity. These may be symptomatic of early stage of lymphedema where accumulation of lymph is mild and not detectable by changes in volume or circumference. As lymphedema progresses, definitive diagnosis is commonly based upon an objective measurement of differences between the affected or at-risk limb at the opposite unaffected limb, e.g. in volume or circumference. No generally accepted criterion is definitively diagnostic, although a volume difference of 200 ml between limbs or a 4-cm difference (at a single measurement site or set intervals along the limb) is often used. Bioimpedance measurement (which measures the amount of fluid in a limb) offers greater sensitivity than existing methods.[22]
Chronic venous stasis changes can mimic early lymphedema, but the changes in venous stasis are more often bilateral and symmetric. Lipedema can also mimic lymphedema, however lipedema characteristically spares the feet beginning abruptly at the medial malleoli (ankle level).[18] As a part of the initial work-up before diagnosing lymphedema, it may be necessary to exclude other potential causes of lower extremity swelling such as renal failure, hypoalbuminemia, congestive heart-failure, protein-losing nephropathy, pulmonary hypertension, obesity, pregnancy and drug-induced edema.[14]
Lymphedema classification
According to the Fifth WHO Expert Committee on Filariasis[23][24] the most common method of classification of lymphedema is as follows: (The same classification method can be used for both primary and secondary lymphedema) The International Society of Lymphology (ISL) Staging System is based solely on subjective symptoms, making it prone to substantial observer bias. Imaging modalities have been suggested as useful adjuncts to the ISL staging to clarify the diagnosis. The lymphedema expert Dr. Ming-Huei Cheng developed a Cheng’s Lymphedema Grading tool to assess the severity of extremity lymphedema based on objective limb measurements and providing appropriate options for management.[25][26][27]
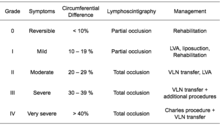
I. Grading
- Grade 1: Spontaneously reversible on elevation. Mostly pitting edema.
- Grade 2: Non-spontaneously reversible on elevation. Mostly non-pitting edema.
- Grade 3: Gross increase in volume and circumference of Grade 2 lymphedema, with eight stages of severity given below based on clinical assessments.
II. Staging
As described by the Fifth WHO Expert Committee on Filariasis,[23][24] and endorsed by the American Society of Lymphology.[28], the staging system helps to identify the severity of lymphedema. With the assistance of medical imaging apparatus, such as MRI or CT, staging can be established by the physician, and therapeutic or medical interventions may be applied:
- Stage 0: The lymphatic vessels have sustained some damage that is not yet apparent. Transport capacity is sufficient for the amount of lymph being removed. Lymphedema is not present.
- Stage 1 : Swelling increases during the day and disappears overnight as the patient lies flat in bed. Tissue is still at the pitting stage: when pressed by the fingertips, the affected area indents and reverses with elevation. Usually upon waking in the morning, the limb or affected area is normal or almost normal in size. Treatment is not necessarily required at this point.
- Stage 2: Swelling is not reversible overnight, and does not disappear without proper management. The tissue now has a spongy consistency and is considered non-pitting: when pressed by the fingertips, the affected area bounces back without indentation. Fibrosis found in Stage 2 lymphedema marks the beginning of the hardening of the limbs and increasing size.
- Stage 3: Swelling is irreversible and usually the limb(s) or affected area become increasingly large. The tissue is hard (fibrotic) and unresponsive; some patients consider undergoing reconstructive surgery, called "debulking". This remains controversial, however, since the risks may outweigh the benefits and the further damage done to the lymphatic system may in fact make the lymphedema worse.
- Stage 4: The size and circumference of the affected limb(s) become noticeably large. Bumps, lumps, or protusions (also called knobs) on the skin begin to appear.
- Stage 5: The affected limb(s) become grossly large; one or more deep skin folds is prevalent among patients in this stage.
- Stage 6: Knobs of small elongated or small rounded sizes cluster together, giving mossy-like shapes on the limb. Mobility of the patient becomes increasingly difficult.
- Stage 7: The patient becomes handicapped, and is unable to independently perform daily routine activities such as walking, bathing and cooking. Assistance from the family and health care system is needed.
Presented below are upper and lower extremity lymphedema between stages 1 to 4:
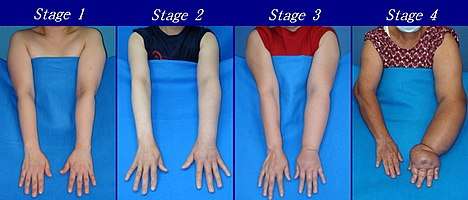 Severity of upper extremity lymphedema in different stages
Severity of upper extremity lymphedema in different stages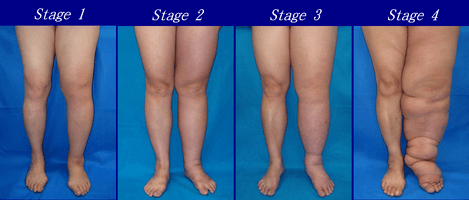 Severity of lower extremity lymphedema in different stages
Severity of lower extremity lymphedema in different stages
Other classification methods
Grades
Lymphedema can also be categorized by its severity (usually referenced to a healthy extremity):[29]
- Grade 1 (mild edema): Involves the distal parts such as a forearm and hand or a lower leg and foot. The difference in circumference is less than 4 cm and other tissue changes are not yet present.
- Grade 2 (moderate edema): Involves an entire limb or corresponding quadrant of the trunk. Difference in circumference is 4–6 cm. Tissue changes, such as pitting, are apparent. The patient may experience erysipelas.
- Grade 3a (severe edema): Lymphedema is present in one limb and its associated trunk quadrant. Circumferential difference is greater than 6 centimeters. Significant skin alterations, such as cornification or keratosis, cysts and/or fistulae, are present. Additionally, the patient may experience repeated attacks of erysipelas.
- Grade 3b (massive edema): The same symptoms as grade 3a, except that two or more extremities are affected.
- Grade 4 (gigantic edema): In this stage of lymphedema, the affected extremities are huge, due to almost complete blockage of the lymph channels.
Treatment
Treatment varies depending on edema severity and the degree of fibrosis. Most people with lymphedema follow a daily regimen of treatment. The most common treatments are a combination of manual compression lymphatic massage, compression garments or bandaging.[18] Complex decongestive physiotherapy is an empiric system of lymphatic massage, skin care and compressive garments. Although a combination treatment program may be ideal, any of the treatments can be done individually. In these last years the Godoy Method brings a new concept in the treatment of lymphedema and proposes the normalization or near normalization in all clinical stages including in elephantiasis with normalization of the skin.[22,23]
Complete decongestive therapy
CDT is a primary tool in lymphedema management.[18] It consists of manual manipulation of the lymphatic ducts,[30][31] short-stretch compression bandaging, therapeutic exercise and skin care. The technique was pioneered by Emil Vodder in the 1930s for the treatment of chronic sinusitis and other immune disorders. Initially, CDT involves frequent visits to a therapist. Once the lymphedema is reduced, increased patient participation is required for ongoing care, along with the use of elastic compression garments and nonelastic directional flow foam garments.
Manual manipulation of the lymphatic ducts (manual lymphatic drainage or MLD) consists of gentle, rhythmic massage to stimulate lymph flow and its return to the blood circulation system. The treatment is gentle. A typical session involves drainage of the neck, trunk and involved extremity (in that order), lasting approximately 40 to 60 minutes. CDT is generally effective on nonfibrotic lymphedema and less effective on more fibrotic legs, although it helps break up fibrotic tissue.
Compression
Garments

Elastic compression garments are worn on the affected limb following complete decongestive therapy to maintain edema reduction. Inelastic garments provide containment and reduction.[18]
Bandaging
Compression bandaging, also called wrapping, is the application of layers of padding and short-stretch bandages to the involved areas. Short-stretch bandages are preferred over long-stretch bandages (such as those normally used to treat sprains), as the long-stretch bandages cannot produce the proper therapeutic tension necessary to safely reduce lymphedema and may in fact end up producing a tourniquet effect. During activity, whether exercise or daily activities, the short-stretch bandages enhance the pumping action of the lymph vessels by providing increased resistance. This encourages lymphatic flow and helps to soften fluid-swollen areas.
A 2002 study showed patients receiving the combined modalities of manual lymphatic drainage (MLD) with complete decongestive therapy (CDT) and pneumatic pumping had a greater overall reduction in limb volume than patients receiving only MLD/CDT.[32]
Intermittent pneumatic compression therapy
Intermittent pneumatic compression therapy (IPC) utilizes a multi-chambered pneumatic sleeve with overlapping cells to promote movement of lymph fluid.[18] Pump therapy should be used in addition to other treatments such as compression bandaging and manual lymph drainage. In some cases, pump therapy helps soften fibrotic tissue and therefore potentially enable more efficient lymphatic drainage. However, reports link pump therapy to increased incidence of edema proximal to the affected limb, such as genital edema arising after pump therapy in the lower limb.[33] IPC should be used in combination with complete decongestive therapy.[32]
Exercise
Most studies investigating the effects exercise in patients with lymphedema or at risk of developing lymphedema examined patients with breast-cancer-related lymphedema. In these studies, resistance training did not increase swelling in patients with pre-existing lymphedema and decreases edema in some patients, in addition to other potential beneficial effects on cardiovascular health.[34][35][36][37] Moreover, resistance training and other forms of exercise were not associated with an increased risk of developing lymphedema in patients who previously received breast cancer-related treatment. Compression garments should be worn during exercise (with the possible exception of swimming in some patients).[38] Patients who have or risk lymphedema should consult their physician or certified lymphedema therapist before beginning an exercise regimen. Resistance training is not recommended in the immediate post-operative period in patients who have undergone axillary lymph node dissection for breast cancer.
Few studies examine the effects of exercise in primary lymphedema or in secondary lymphedema that is not related to breast cancer treatment.
Surgery
Several surgical procedures provide long-term solutions for patients who suffer from lymphedema. Prior to surgery, patients typically are treated by a physical or an occupational therapist trained in providing lymphedema treatment for initial conservative treatment of their lymphedema. CDT, MLD and compression bandaging are all helpful components of conservative lymphedema treatment.[39]
Vascularized lymph node transfer
Vascularized lymph node transfers (VLNT) can be an effective treatment of the arm and upper extremity. Lymph nodes are harvested from the groin area or the supraclavicular area with their supporting artery and vein and moved to the axilla (armpit) or the wrist area. Microsurgery techniques connect the artery and vein to blood vessels in the axilla to provide support to the lymph nodes while they develop their own blood supply over the first few weeks after surgery.
The newly transferred lymph nodes then serve as a conduit or filter to remove the excess lymphatic fluid from the arm and return it to the body's natural circulation.
This technique of lymph node transfer may be performed together with a DIEP flap breast reconstruction. This allows for both the simultaneous treatment of the arm lymphedema and the creation of a breast in one surgery. The lymph node transfer removes the excess lymphatic fluid to return form and function to the arm. In selected cases, the lymph nodes may be transferred as a group with their supporting artery and vein, but without the associated abdominal tissue for breast reconstruction.
Lymph node transfers are most effective in patients whose extremity circumference reduces significantly with compression wrapping, indicating most of the edema is fluid.
VLNT significantly improves the fluid component of lymphedema and decrease the amount of lymphedema therapy and compression garment use required.[40]
Lymphaticovenous anastomosis
Lymphaticovenous anastomosis (LVA) uses supermicrosurgery to connect the affected lymphatic channels directly to tiny veins located nearby. The lymphatics are tiny, typically 0.1 mm to 0.8 mm in diameter. The procedure requires the use of specialized techniques with superfine surgical suture and an adapted, high-power microscope.
LVA can be an effective and long-term solution for extremity lymphedema and many patients have results that range from a moderate improvement to an almost complete resolution. LVA is most effective early in the course of the disease in patients whose extremity circumference reduces significantly with compression wrapping, indicating most of the edema is fluid. Patients who do not respond to compression are less likely to fare well with LVA, as a greater amount of their increased extremity volume consists of fibrotic tissue, protein or fat. Multiple studies showed LVAs to be effective.[40][41][42]
Lymphaticovenous anastomosis was introduced by B. M. O'Brien and colleagues for the treatment of obstructive lymphedema in the extremities.[43] In 2003, supermicrosurgery pioneer Isao Koshima and colleagues improved the surgery with supermicrosurgical techniques and established the new standard in reconstructive microsurgery.[43] Studies involving long-term follow-up after LVA for lymphedema indicated patients showed remarkable improvement compared to conservative treatment using continuous elastic stocking and occasional pumping.[43]
Clinical studies involving LVA indicate immediate and long-term results showed significant reductions in volume and improvement in systems that appear to be long-lasting.[40][44][45] A 2006 study compared two groups of breast cancer patients at high risk for lymphedema in whom LVA was used to prevent the onset of clinically evident lymphedema. Results showed a statistically significant reduction in the number of patients who went on to develop clinically significant lymphedema.[45] Other studies showed LVA surgeries reduce the severity of lymphedema in breast cancer patients.[46][47] In particular, a clinical study of 1,000 cases of lymphedema treated with microsurgery from 1973 to 2006 showed beneficial results.[47] Clinical reports from microsurgeons and physical therapists documented more than 1,500 patients treated with LVA surgery over a span of 30 years showing significant improvement and effectiveness.[42]
Indocyanine green fluoroscopy is a safe, minimally invasive and useful tool for surgical evaluation.[48] Microsurgeons use indocyanine green lymphography to assist in LVA surgeries.[49]
Suction assisted lipectomy
People whose limbs no longer adequately respond to compression therapy may be candidates for suction assisted lipectomy (SAL). This procedure has been called liposuction for lymphedema and is specifically adapted to treat this advanced condition. SAL employs a different operative technique and requires significant therapy and compression garment care that must be administered by a therapist experienced in the technique.
This procedure was pioneered by Hakan Brorson in 1987.[7] Well-controlled clinical trials conducted from 1993 to 2014 showed SAL, combined with controlled compression therapy (CCT), to be an effective lymphedema treatment without recurrence.[7][40][41][50][51][52][53][54] Long-term followup (11–13 years) of patients with lymphedema showed no recurrence of swelling.[7] Lymphatic liposuction combined with controlled compression therapy was more effective than controlled compression therapy alone.[55][56]
SAL has been refined in recent years by using vibrating cannulae that are finer and more effective than previous equipment.[7] In addition, the introduction of the tourniquet and tumescent technique led to minimized blood loss.[7][57]
SAL uses specialized techniques that differ from conventional liposuction procedures and requires specific training.
Lymphatic vessel grafting
With advanced microsurgical techniques, lymph vessels can be used as grafts. A locally interrupted or obstructed lymphatic pathway, mostly after resection of lymph nodes, can be reconstructed via a bypass using lymphatic vessels. These vessels are specialized to drain lymph by active pumping forces. These grafts are connected with main lymphatic collectors in front and behind the obstruction. The technique is mostly used in arm edemas after treatment of breast cancer and in unilateral edemas of lower extremities after resection of lymph nodes and radiation. The procedure is less widely used than the other surgical procedures, mainly in Germany. The method was developed in 1980 by Ruediger Baumeister.[58]
The method is proven effective.[59] Follow-up studies showed significant volume reduction of the extremities even 10 years after surgery.[60] The patients, who had been previously treated with both MLD and compression therapy, gained significant improvement in quality of life after being treated with lymphatic vessel grafting.[61] Lymphoscintigraphic investigations showed a lasting enhancement of lymphatic transport after grafting.[62]
The patency of lymphatic grafts was demonstrated after more than 12 years, using indirect lymphography and MRI lymphography.
Low level laser therapy
Low-level laser therapy (LLLT) was cleared by the US Food and Drug Administration (FDA) for the treatment of lymphedema in November 2006.[63]
According to the US National Cancer Institute,
Studies suggest that low-level laser therapy may be effective in reducing lymphedema in a clinically meaningful way for some women. Two cycles of laser treatment were found to be effective in reducing the volume of the affected arm, extracellular fluid, and tissue hardness in approximately one-third of patients with postmastectomy lymphedema at 3 months post-treatment. Suggested rationales for laser therapy include a potential decrease in fibrosis, stimulation of macrophages and the immune system, and a possible role in encouraging lymphangiogenesis.[64][65]
Prevalence
Lymphedema affects approximately 200 million people worldwide.[1]
See also
References
- 1 2 3 4 5 6 Grada, Ayman A.; Phillips, Tania J. (2017). "Lymphedema: Pathophysiology and clinical manifestations". Journal of the American Academy of Dermatology. 77 (6): 1009–1020. doi:10.1016/j.jaad.2017.03.022. ISSN 1097-6787. PMID 29132848.
- ↑ Martin MB, Kon ND, Kawamoto EH, Myers RT, Sterchi JM (1984). "Postmastectomy angiosarcoma". The American surgeon. 50 (10): 541–5. PMID 6541442.
- ↑ Chopra S, Ors F, Bergin D (2007). "MRI of angiosarcoma associated with chronic lymphoedema: Stewart Treves syndrome". British Journal of Radiology. 80 (960): e310–3. doi:10.1259/bjr/19441948. PMID 18065640.
- ↑ Requena L, Sangueza OP (1998). "Cutaneous vascular proliferations. Part III. Malignant neoplasms, other cutaneous neoplasms with significant vascular component, and disorders erroneously considered as vascular neoplasms". Journal of the American Academy of Dermatology. 38 (2): 143–75, quiz 176–8. doi:10.1016/S0190-9622(98)70237-3. PMID 9486670.
- ↑ Jeannie Burt; Gwen White (1 January 2005). Lymphedema: A Breast Cancer Patient's Guide to Prevention and Healing. Hunter House. p. 9. ISBN 978-0-89793-458-9.
- ↑ Choices, NHS. "IPS retired" (PDF). www.nhs.uk. Retrieved 9 May 2018.
- 1 2 3 4 5 6 7 8 9 Brorson H, Ohlin K, Olsson G, Svensson B, Svensson H (June 2008). "Controlled compression and liposuction treatment for lower extremity lymphedema". Lymphology. 41 (2): 52–63. PMID 18720912.
- ↑ Kissin MW, Querci della Rovere G, Easton D, Westbury G (July 1986). "Risk of lymphoedema following the treatment of breast cancer". Br J Surg. 73 (7): 580–4. doi:10.1002/bjs.1800730723. PMID 3730795.
- ↑ Segerström K, Bjerle P, Graffman S, Nyström A (1992). "Factors that influence the incidence of brachial oedema after treatment of breast cancer". Scand J Plast Reconstr Surg Hand Surg. 26 (2): 223–7. doi:10.3109/02844319209016016. PMID 1411352.
- ↑ Werngren-Elgström M, Lidman D (December 1994). "Lymphoedema of the lower extremities after surgery and radiotherapy for cancer of the cervix". Scand J Plast Reconstr Surg Hand Surg. 28 (4): 289–93. doi:10.3109/02844319409022014. PMID 7899840.
- ↑ Pilepich MV, Asbell SO, Mulholland GS, Pajak T (1984). "Surgical staging in carcinoma of the prostate: the RTOG experience. Radiation Therapy Oncology Group". Prostate. 5 (5): 471–6. doi:10.1002/pros.2990050502. PMID 6483687.
- ↑ Pilepich MV, Krall J, George FW, Asbell SO, Plenk HD, Johnson RJ, Stetz J, Zinninger M, Walz BJ (1994). "Treatment-related morbidity in Phase III RTOG studies of extended-field irradiation for carcinoma of the prostate". Int. J. Radiat. Oncol. Biol. Phys. 10 (10): 1861–7. doi:10.1016/0360-3016(84)90263-3. PMID 6386761.
- ↑ Liem, Timothy K.; Moneta, Gregory L. (2010). Brunicardi, F. Charles; Andersen, Dana K.; Billiar, Timothy R.; Dunn, David L.; Hunter, John G.; Matthews, Jeffrey B.; Pollock, Raphael E., eds. Schwartz's Principles of Surgery (9 ed.). New York, NY: The McGraw-Hill Companies.
- 1 2 Burkhart, Craig N.; Adigun, Chris; Burton, Claude S. (2012). Goldsmith, Lowell A.; Katz, Stephen I.; Gilchrest, Barbara A.; Paller, Amy S.; Leffell, David J.; Wolff, Klaus, eds. Fitzpatrick's Dermatology in General Medicine (8 ed.). New York, NY: The McGraw-Hill Companies.
- ↑ Crispino JD, Horwitz MS (April 2017). "GATA factor mutations in hematologic disease". Blood. 129 (15): 2103–2110. doi:10.1182/blood-2016-09-687889. PMC 5391620. PMID 28179280.
- ↑ Hirabayashi S, Wlodarski MW, Kozyra E, Niemeyer CM (August 2017). "Heterogeneity of GATA2-related myeloid neoplasms". International Journal of Hematology. 106 (2): 175–182. doi:10.1007/s12185-017-2285-2. PMID 28643018.
- ↑ Kurland, L. T.; Molgaard, C. A. (October 1981). "The patient record in epidemiology". Scientific American. 245 (4): 54–63. ISSN 0036-8733. PMID 7027437.
- 1 2 3 4 5 6 Grada, Ayman A.; Phillips, Tania J. (December 2017). "Lymphedema: Diagnostic workup and management". Journal of the American Academy of Dermatology. 77 (6): 995–1006. doi:10.1016/j.jaad.2017.03.021. ISSN 1097-6787. PMID 29132859.
- ↑ Burnand, Katherine M.; Glass, Daphne M.; Mortimer, Peter S.; Peters, Adrien Michael (January 2012). "Lymphatic dysfunction in the apparently clinically normal contralateral limbs of patients with unilateral lower limb swelling". Clinical Nuclear Medicine. 37 (1): 9–13. doi:10.1097/RLU.0b013e31823931f5. ISSN 1536-0229. PMID 22157021.
- ↑ Tiwari, Alok; Cheng, Koon-Sung; Button, Matthew; Myint, Fiona; Hamilton, George (February 2003). "Differential diagnosis, investigation, and current treatment of lower limb lymphedema". Archives of Surgery (Chicago, Ill.: 1960). 138 (2): 152–161. ISSN 0004-0010. PMID 12578410.
- 1 2 Jarvis, C. (2004). Physical Examination and Health Assessment (5th ed.). Saunders Elsevier. pp. 530–553. ISBN 1-4160-5188-0.
- ↑ Ward LC (2006). "Bioelectrical Impedance Analysis: Proven Utility in Lymphedema Risk Assessment and Therapeutic Monitoring". Lymphatic Research and Biology. 4 (1): 51–6. doi:10.1089/lrb.2006.4.51. PMID 16569209.
- 1 2 "Treatment and Prevention of Problems Associated with Lymphatic Filariasis" (PDF). World Health Organization. Archived (PDF) from the original on 2012-04-18. Retrieved 2014-05-16.
- 1 2 "Lymphatic filariasis: The disease and its control. Fifth report of the WHO Expert Committee on Filariasis". World Health Organization technical report series. 821: 1–71. 1992. PMID 1441569.
- ↑ International Society of Lymphology (March 2013). "The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology". Lymphology. 46 (1): 1–11. ISSN 0024-7766. PMID 23930436.
- ↑ Patel, Ketan M.; Lin, Chia-Yu; Cheng, Ming-Huei (July 2015). "A Prospective Evaluation of Lymphedema-Specific Quality-of-Life Outcomes Following Vascularized Lymph Node Transfer". Annals of Surgical Oncology. 22 (7): 2424–2430. doi:10.1245/s10434-014-4276-3. ISSN 1534-4681. PMID 25515196.
- ↑ Cheng, Ming-Huei; Chang, David W.; Patel, Ketan M. (13 July 2015). Principles and Practice of Lymphedema Surgery. Elsevier Health Sciences. ISBN 978-0-323-29897-1.
- ↑ Tretbar, Lawrence L; Morgan, Cheryl L.; Lee, Byung-Boong; Simonian, Simon J.; Blondeau, Benoit (6 May 2010). Lymphedema: Diagnosis and Treatment. Springer Science & Business Media. ISBN 978-1-84628-793-0.
- ↑ Lee, Teresa S.; Morris, Carol M.; Czerniec, Sharon A.; Mangion, Andrea J. (1 February 2018). "Does Lymphedema Severity Affect Quality of Life? Simple Question. Challenging Answers". Lymphatic Research and Biology. 16 (1): 85–91. doi:10.1089/lrb.2016.0049. PMID 28453410.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 2007-08-23. Retrieved 2007-09-01.
- ↑ NLN Medical Advisory Committee (February 2011). "The Diagnosis And Treatment Of Lymphedema" (PDF). www.lymphnet.org. National Lymphedema Network. Archived (PDF) from the original on 2017-04-28.
- 1 2 Szuba A, Achalu R, Rockson SG (2002). "Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema". Cancer. 95 (11): 2260–7. doi:10.1002/cncr.10976. PMID 12436430.
- ↑ Boris M, Weindorf S, Lasinski BB (Mar 1998). "The risk of genital edema after external pump compression for lower limb lymphedema". Lymphology. 31 (1): 15–20. PMID 9561507.
- ↑ Markes M, Brockow T, Resch KL (2006). "Exercise for women receiving adjuvant therapy for breast cancer". The Cochrane Database of Systematic Reviews (4): CD005001. doi:10.1002/14651858.CD005001.pub2. PMID 17054230.
- ↑ McKenzie DC, Kalda AL (2003). "Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: A pilot study". Journal of Clinical Oncology. 21 (3): 463–6. doi:10.1200/jco.2003.04.069. PMID 12560436.
- ↑ Ahmed RL, Thomas W, Yee D, Schmitz KH (2006). "Randomized controlled trial of weight training and lymphedema in breast cancer survivors". Journal of Clinical Oncology. 24 (18): 2765–72. doi:10.1200/JCO.2005.03.6749. PMID 16702582.
- ↑ Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP (2009). "Weight lifting in women with breast-cancer-related lymphedema". New England Journal of Medicine. 361 (7): 664–73. doi:10.1056/NEJMoa0810118. PMID 19675330.
- ↑ "Position Paper: Exercise | National Lymphedema Network". Lymphnet.org. Archived from the original on 2014-05-08. Retrieved 2014-05-16.
- ↑ Granzow, Jay W.; Soderberg, Julie M.; Kaji, Amy H.; Dauphine, Christine (2014). "Review of Current Surgical Treatments for Lymphedema". Annals of Surgical Oncology. 21 (4): 1195–1201. doi:10.1245/s10434-014-3518-8. ISSN 1068-9265.
- 1 2 3 4 Granzow, Jay W.; Soderberg, Julie M.; Kaji, Amy H.; Dauphine, Christine (2014). "An Effective System of Surgical Treatment of Lymphedema". Annals of Surgical Oncology. 21 (4): 1189–1194. doi:10.1245/s10434-014-3515-y. ISSN 1068-9265.
- 1 2 Granzow JW, Soderberg JM, Dauphine C. A Novel Two-Stage Surgical Approach to Treat Chronic Lymphedema. Breast J. 2014 Jun 19.
- 1 2 Campisi C, Eretta C, Pertile D, Da Rin E, Campisi C, Macciò A, Campisi M, Accogli S, Bellini C, Bonioli E, Boccardo F (2007). "Microsurgery for treatment of peripheral lymphedema: long-term outcome and future perspectives". Microsurgery. 27 (4): 333–8. doi:10.1002/micr.20346. PMID 17477420.
- 1 2 3 Koshima I, Nanba Y, Tsutsui T, Takahashi Y, Itoh S (May 2003). "Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg". J Reconstr Microsurg. 19 (4): 209–15. doi:10.1055/s-2003-40575. PMID 12858242.
- ↑ Granzow, Jay W.; Soderberg, Julie M.; Dauphine, Christine (July 2014). "A novel two-stage surgical approach to treat chronic lymphedema". The Breast Journal. 20 (4): 420–422. doi:10.1111/tbj.12282. ISSN 1524-4741. PMID 24943048.
- 1 2 Campisi C, Davini D, Bellini C, Taddei G, Villa G, Fulcheri E, Zilli A, da Rin E, Eretta C, Boccardo F (2006). "Is there a role for microsurgery in the prevention of arm lymphedema secondary to breast cancer treatment?". Microsurgery. 26 (1): 70–2. doi:10.1002/micr.20215. PMID 16444710.
- ↑ Chang DW (September 2010). "Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study". Plast. Reconstr. Surg. 126 (3): 752–8. doi:10.1097/PRS.0b013e3181e5f6a9. PMID 20811210.
- 1 2 Campisi C, Davini D, Bellini C, Taddei G, Villa G, Fulcheri E, Zilli A, Da Rin E, Eretta C, Boccardo F (2006). "Lymphatic microsurgery for the treatment of lymphedema". Microsurgery. 26 (1): 65–9. doi:10.1002/micr.20214. PMID 16444753.
- ↑ Yamamoto T, Narushima M, Doi K, Oshima A, Ogata F, Mihara M, Koshima I, Mundinger GS (May 2011). "Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns". Plast. Reconstr. Surg. 127 (5): 1979–86. doi:10.1097/PRS.0b013e31820cf5df. PMID 21532424.
- ↑ Ogata F, Narushima M, Mihara M, Azuma R, Morimoto Y, Koshima I (August 2007). "Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema". Ann Plast Surg. 59 (2): 180–4. doi:10.1097/01.sap.0000253341.70866.54. PMID 17667413.
- ↑ Brorson, Hakan; Karin Ohlin; Barbro Svensson (2008). "The Facts About Liposuction As A Treatment For Lymphoedema". Journal of Lymphoedema. 3 (1): 38–47. Archived from the original on 2018-03-14.
- ↑ Brorson H, Svensson H (June 1997). "Complete reduction of lymphoedema of the arm by liposuction after breast cancer". Scand J Plast Reconstr Surg Hand Surg. 31 (2): 137–43. doi:10.3109/02844319709085480. PMID 9232698.
- ↑ Brorson H (2000). "Liposuction gives complete reduction of chronic large arm lymphedema after breast cancer". Acta Oncol. 39 (3): 407–20. doi:10.1080/028418600750013195. PMID 10987239.
- ↑ Brorson H (2003). "Liposuction in arm lymphedema treatment" (PDF). Scand J Surg. 92 (4): 287–95. PMID 14758919.
- ↑ Brorson, H.; K. Ohlin; G. Olsson; et al. (2006). "Long term cosmetic and functional results following liposuction for arm lymphedema: An eleven year study". Lymphology. 40 (Supp): 253–255. Search of journal did not yield an url
- ↑ Brorson H, Svensson H (September 1998). "Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone". Plast. Reconstr. Surg. 102 (4): 1058–67, discussion 1068. doi:10.1097/00006534-199809020-00022. PMID 9734424.
- ↑ Damstra RJ, Voesten HG, Klinkert P, Brorson H (August 2009). "Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer". Br J Surg. 96 (8): 859–64. doi:10.1002/bjs.6658. PMID 19591161.
- ↑ Wojnikow S, Malm J, Brorson H (2007). "Use of a tourniquet with and without adrenaline reduces blood loss during liposuction for lymphoedema of the arm". Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 41 (5): 243–9. doi:10.1080/02844310701546920. PMID 17886128.
- ↑ Baumeister RG, Seifert J, Wiebecke B, Hahn D (May 1981). "Experimental basis and first application of clinical lymph vessel transplantation of secondary lymphedema". World J Surg. 5 (3): 401–7. doi:10.1007/BF01658013. PMID 7293201.
- ↑ Baumeister RG, Siuda S (January 1990). "Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved?". Plast. Reconstr. Surg. 85 (1): 64–74, discussion 75–6. doi:10.1097/00006534-199001000-00012. PMID 2293739.
- ↑ Baumeister RG, Frick A (July 2003). "[The microsurgical lymph vessel transplantation]". Handchir Mikrochir Plast Chir (in German). 35 (4): 202–9. doi:10.1055/s-2003-42131. PMID 12968216.
- ↑ Springer S, Koller M, Baumeister RG, Frick A (June 2011). "Changes in quality of life of patients with lymphedema after lymphatic vessel transplantation". Lymphology. 44 (2): 65–71. PMID 21949975.
- ↑ Weiss M, Baumeister RG, Hahn K (November 2002). "Post-therapeutic lymphedema: scintigraphy before and after autologous lymph vessel transplantation: 8 years of long-term follow-up". Clin Nucl Med. 27 (11): 788–92. doi:10.1097/01.RLU.0000033613.05410.34. PMID 12394126.
- ↑ dotmed.com December 27, 2006 Archived January 7, 2010, at the Wayback Machine. Low Level Laser FDA Cleared for the Treatment of Lymphedema. (accessed 9 November 09)
- ↑ National Cancer Institute: Low-level laser therapy Archived 2009-09-24 at the Wayback Machine. accessed 9 November 09
- ↑ Carati CJ, Anderson SN, Gannon BJ, Piller NB (2003). "Treatment of postmastectomy lymphedema with low-level laser therapy". Cancer. 98 (6): 1114–22. doi:10.1002/cncr.11641. PMID 12973834.
22-Pereira de Godoy JM, Pereira de Godoy HJ, Lopes Pinto R, Facio FN Jr, Guerreiro Godoy MF.Maintenance of the Results of Stage II Lower Limb Lymphedema Treatment after Normalization of Leg Size.Int J Vasc Med. 2017;2017:8515767. doi: 10.1155/2017/8515767. Epub 2017 Aug 1.
23-Pereira de Godoy HJ, Budtinger Filho R, Godoy MF, de Godoy JM.Evolution of Skin during Rehabilitation for Elephantiasis Using Intensive Treatment.Case Rep Dermatol Med. 2016;2016:4305910. doi: 10.1155/2016/4305910. Epub 2016 Nov 24.
External links
| Classification | |
|---|---|
| External resources |
| Wikimedia Commons has media related to Lymphedema. |