Locked nucleic acid
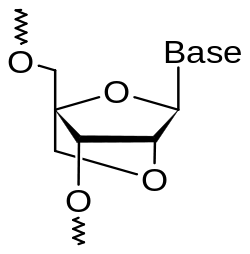
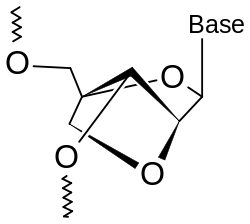
A locked nucleic acid (LNA), often referred to as inaccessible RNA, is a modified RNA nucleotide. The ribose moiety of an LNA nucleotide is modified with an extra bridge connecting the 2' oxygen and 4' carbon. The bridge "locks" the ribose in the 3'-endo (North) conformation, which is often found in the A-form duplexes. LNA nucleotides can be mixed with DNA or RNA residues in the oligonucleotide whenever desired and hybridize with DNA or RNA according to Watson-Crick base-pairing rules. Such oligomers are synthesized chemically and are commercially available. The locked ribose conformation enhances base stacking and backbone pre-organization. This significantly increases the hybridization properties (melting temperature) of oligonucleotides.[1] [2] LNA was independently synthesized by the group of Jesper Wengel[3] in 1998, soon after the first synthesis by the group of Takeshi Imanishi.[4]
Using LNA based oligonucleotides therapeutically is an emerging field in biotechnology. The Danish pharmaceutical company Santaris Pharma a/s owns the sole rights to therapeutic uses of LNA technology,[5] and is now developing a new, LNA based, hepatitis C drug called miravirsen, targeting miR-122, which is in Phase II clinical testing as of late 2010.[6]
Allele-specific PCR using LNA allows for the design of shorter primers, without compromising binding specificity [7]
Notes and references
- ↑ Kaur, H; Arora, A; Wengel, J; Maiti, S (2006). "Thermodynamic, Counterion, and Hydration Effects for the Incorporation of Locked Nucleic Acid Nucleotides into DNA Duplexes". Biochemistry. 45 (23): 7347–55. doi:10.1021/bi060307w. PMID 16752924.
- ↑ Owczarzy R.; You Y.; Groth C.L.; Tataurov A.V. (2011). "Stability and mismatch discrimination of locked nucleic acid-DNA duplexes". Biochemistry. 50 (43): 9352–9367. doi:10.1021/bi200904e. PMC 3201676. PMID 21928795.
- ↑ Alexei A. Koshkin; Sanjay K. Singh; Poul Nielsen; Vivek K. Rajwanshi; Ravindra Kumar; Michael Meldgaard; Carl Erik Olsen; Jesper Wengel (1998). "LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition". Tetrahedron. 54 (14): 3607–30. doi:10.1016/S0040-4020(98)00094-5.
- ↑ Satoshi Obika; Daishu Nanbu; Yoshiyuki Hari; Ken-ichiro Morio; Yasuko In; Toshimasa Ishida; Takeshi Imanishi (1997). "Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3'-endo sugar puckering". Tetrahedron Lett. 38 (50): 8735–8. doi:10.1016/S0040-4039(97)10322-7.
- ↑ "Developing LNA technology for new-generation cancer drugs" (PDF). SP2 Magazine. March 2006.
- ↑ Franciscus, Alan (2010). "Hepatitis C Treatments in Current Development". HCV Advocate. Archived from the original on 2003-09-06.
- ↑ Bonetta, Laura (2005). "Prime time for real-time PCR". Nat. Methods. 2 (4): 305–312. doi:10.1038/nmeth0405-305.
External links
- LNA Oligo melting temperature including mismatches
- Petersen M, Wengel J (February 2003). "LNA: a versatile tool for therapeutics and genomics". Trends Biotechnol. 21 (2): 74–81. doi:10.1016/S0167-7799(02)00038-0. PMID 12573856.