Lead(IV) acetate
 | |
| Names | |
|---|---|
| IUPAC name
Lead(IV) acetate | |
| Other names
Lead tetraacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ECHA InfoCard | 100.008.099 |
PubChem CID |
|
| |
| |
| Properties | |
| Pb(C2H3O2)4 | |
| Molar mass | 443.376 g/mol |
| Appearance | colorless or pink crystals |
| Odor | vinegar |
| Density | 2.228 g/cm3 (17 °C) |
| Melting point | 175 °C (347 °F; 448 K) |
| Boiling point | decomposes |
| soluble, reversible hydrolysis | |
| Solubility | reacts with ethanol soluble in chloroform, benzene, nitrobenzene, hot acetic acid, HCl, tetrachloroethane |
| Hazards | |
| Main hazards | Toxic |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lead(IV) acetate or lead tetraacetate is a chemical compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar organic solvents, indicative that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.[1]
Structure
In the solid state the lead(IV) centers are coordinated by four acetate ions, which are bidentate, each coordinating via two oxygen atoms. The lead atom is 8 coordinate and the O atoms form a flattened trigonal dodecahedron.[2]
Preparation
It is typically prepared by treating of red lead with acetic acid and acetic anhydride (Ac2O), which absorbs water. The net reaction is shown:[3]
- Pb3O4 + 4 Ac2O → Pb(OAc)4 + 2 Pb(OAc)2
The remaining lead(II) acetate can be partially oxidized to the tetraacetate:
- 2 Pb(OAc)2 + Cl2 → Pb(OAc)4 + PbCl2
Procedure
Glacial acetic acid and acetic anhydride are first mixed and heated at 60°C in presence of chlorine gas and small amount of lead powder (about 15-20 g) is added and stirred until the colour is faded. Once the colour is faded, add further lead powder (up to 700 grams) in portions. Then add charcoal to it and filter to get lead chloride and lead tetra acetate. The residue can be filtered twice with warm acetic acid and filtered. Both the filtered solution are then kept aside overnight for the needle shaped crystals of lead acetate to form.
Reagent in organic chemistry
Lead tetraacetate is a strong oxidizing agent,[4] a source of acetyloxy groups and a general reagent for the introduction of lead into organolead compounds. Some of its many uses in organic chemistry:
- acetoxylation of benzylic, allylic and α-oxygen ether C-H bonds, for example the photochemical conversion of dioxane to 1,4-dioxene through the 2-acetoxy-1,4-dioxane intermediate [5] and the conversion of α-pinene to verbenone [6]
- an alternative reagent to bromine in Hofmann rearrangement[7]
- oxidation of hydrazones to diazo compounds for example that of hexafluoroacetone hydrazone to bis(trifluoromethyl)diazomethane [8]
- aziridine formation, for example the reaction of N-aminophthalimide and stilbene [9]
- cleavage of α-hydroxy acids[10] or 1,2-diols to their corresponding aldehydes or ketones, often replacing ozonolysis; for instance, the oxidation of di-n-butyl d-tartrate to n-butyl glyoxylate.[11]
- reaction with alkenes to form γ-lactones
- oxidation of alcohols carrying a δ-proton to cyclic ethers.[12]
- Oxidative cleavage of certain allyl alcohols in conjunction with ozone:[13][14]
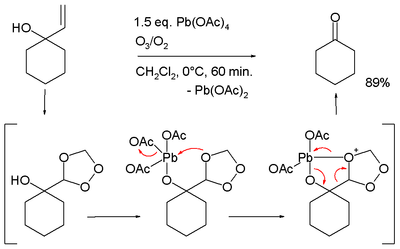
- conversion of acetophenones to phenyl acetic acids [15]
- Decarboxylation of carboxylic acids to alkyl halides in the Kochi reaction[16]
Safety
Lead(IV) acetate may be fatal if ingested, inhaled, or absorbed through skin. It causes irritation to skin, eyes, and respiratory tract. It is a neurotoxin. It affects the gum tissue, central nervous system, kidneys, blood, and reproductive system.
References
- ↑ Mihailo Lj. Mihailović, Živorad Čeković, Brian M. Mathes (2005). "Lead(IV) Acetate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rl006.pub2.
- ↑ Schürmann, M.; Huber, F. (1994). "A redetermination of lead(IV) acetate". Acta Crystallographica Section C. 50 (11): 1710–1713. doi:10.1107/S0108270194006438. ISSN 0108-2701.
- ↑ J. C. Bailar, Jr. (1939). "Lead Tetracetate". Inorganic Syntheses. 1: 47–49. doi:10.1002/9780470132326.ch17.
- ↑ J. Zýka (1966). "Analytical study of the basic properties of lead tetraacetate as oxidizing agent" (PDF). Pure and Applied Chemistry. 13 (4): 569–581. doi:10.1351/pac196613040569. Retrieved 19 December 2013.
- ↑ Organic Syntheses, Vol. 82, p.99 (2005) Article.
- ↑ Organic Syntheses, Coll. Vol. 9, p.745 (1998); Vol. 72, p.57 (1995) Article
- ↑ Baumgarten, Henry; Smith, Howard; Staklis, Andris (1975). "Reactions of amines. XVIII. Oxidative rearrangement of amides with lead tetraacetate". The Journal of Organic Chemistry. 40 (24): 3554–3561. doi:10.1021/jo00912a019. Retrieved 19 December 2013.
- ↑ Organic Syntheses, Coll. Vol. 6, p.161 (1988); Vol. 50, p.6 (1970) Article.
- ↑ Organic Syntheses, Coll. Vol. 6, p.56 (1988); Vol. 55, p.114 (1976) Link
- ↑ Ôeda, Haruomi (1934). "Oxidation of some α-hydroxy-acids with lead tetraacetate". Bulletin of the Chemical Society of Japan. 9 (1): 8–14. doi:10.1246/bcsj.9.8.
- ↑ Organic Syntheses, Coll. Vol. 4, p.124 (1963); Vol. 35, p.18 (1955) Article.
- ↑ M B Smith, J March. March's Advanced Organic Chemistry (Wiley, 2001) ( ISBN 0-471-58589-0)
- ↑ O3/Pb(OAc)4: a new and efficient system for the oxidative cleavage of allyl alcohols E.J. Alvarez-Manzaneda R. Chahboun , M.J. Cano, E. Cabrera Torres, E. Alvarez, R. Alvarez-Manzaneda, b, A. Haidour and J.M. Ramos López Tetrahedron Letters Volume 47, Issue 37 , 11 September 2006, Pages 6619-6622 doi:10.1016/j.tetlet.2006.07.020
- ↑ Conversion of 1-allyl-cyclohexanol to cyclohexanone, in the proposed reaction mechanism the allyl group is first converted to a trioxalane according to conventional ozonolysis which then interacts with the alkoxy lead group
- ↑ "One-Step Synthesis of Methyl Arylacetates from Acetophenones Using Lead(IV) Acetate". Synthesis. 2: 126–127. 1981. doi:10.1055/s-1981-29358.
- ↑ Jay K. Kochi (1965). "A New Method for Halodecarboxylation of Acids Using Lead(IV) Acetate". J. Am. Chem. Soc. 87: 2500–02. doi:10.1021/ja01089a041.
Acetyl halides and salts of the acetate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 | Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
