Kleiber's law
Kleiber's law,[1] named after Max Kleiber for his biology work in the early 1930s, is the observation that, for the vast majority of animals, an animal's metabolic rate scales to the ¾ power of the animal's mass. Symbolically: if q0 is the animal's metabolic rate, and M the animal's mass, then Kleiber's law states that q0 ~ M¾. Thus, over the same timespan, a cat having a mass 100 times that of a mouse will consume only about 32 times the energy the mouse uses. In plants, the exponent is close to 1.
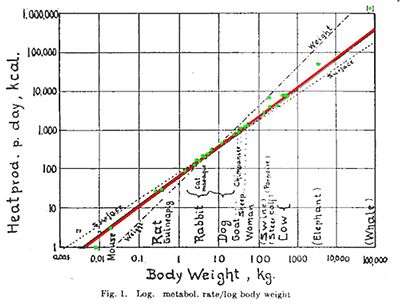
Biography
Max Kleiber[3] was born and educated in Zürich, Switzerland. He graduated from the Federal Institute of Technology as an Agricultural Chemist in 1920, earned the ScD degree in 1924, and became a private dozent after publishing his thesis The Energy Concept in the Science of Nutrition.
He came to the Animal Husbandry Department of UC Davis in 1929 to construct respiration chambers and conduct research on energy metabolism in animals. Among his many important achievements, two are especially noteworthy. In 1932 he came to the conclusion that the ¾ power of body weight was the most reliable basis for predicting the basal metabolic rate (BMR) of animals and for comparing nutrient requirements among animals of different size. He also provided the basis for the conclusion that total efficiency of energy utilization is independent of body size. These concepts and several others fundamental for understanding energy metabolism are discussed in Kleiber's book, The Fire of Life published in 1961 and subsequently translated into German, Polish, Spanish, and Japanese.
Reasoning behind the law and value of the exponent
Kleiber's law, as many other biological allometric laws, is a consequence of the physics and/or geometry of animal circulatory systems, according to some authors.[4][5][6] Young (i.e., small) organisms respire more per unit of weight than old (large) ones of the same species because of the overhead costs of growth, but small adults of one species respire more per unit of weight than large adults of another species because a larger fraction of their body mass consists of structure rather than reserve; structural mass involves maintenance costs, reserve mass does not.
The exponent for Kleiber's law, which is called a power law, was a matter of dispute for many decades. It is still contested by a diminishing number as being 2⁄3 rather than the more widely accepted 3⁄4. Because the law concerned the capture, use, and loss of energy by a biological system, the system's metabolic rate was, at first, taken to be 2⁄3, because energy was thought of mostly in terms of heat energy. Metabolic rate was expressed in energy per unit time, specifically calories per second. Two thirds expressed the relation of the square of the radius to the cube of the radius of a sphere, with the volume of the sphere increasing faster than the surface area, with increases in radius. This was purportedly the reason large creatures lived longer than small ones – that is, as they got bigger they lost less energy per unit volume through the surface, as radiated heat.
The problem with 2⁄3 as an exponent was that it did not agree with a lot of the data. There were many exceptions, and the concept of metabolic rate itself was poorly defined and difficult to measure. It seemed to concern more than rate of heat generation and loss. Since what was being considered was not necessarily Euclidean geometry, the appropriateness of 2⁄3 as an exponent was questioned. Kleiber himself came to favor 3⁄4, and that is the number favored today by the foremost proponents of the law, despite that 3⁄4 also does not agree with much of the data, and is also troubled with exceptions. For example, although roughly the mammal data fit with 3⁄4 for the whole range of masses, when restricting to masses under 10 kg they fit better to 2⁄3.[7] In fact, mammals data are not a pure power law as they show a clear curvature.[8] Low mass endotherms as birds also fit better to 2⁄3 as an exponent, but ectotherms as insects fit better to 1. Theoretical models presented by Geoffrey West, Brian Enquist, and James Brown,[9] – known as the WBE model – purport to show how the 3⁄4 observation can emerge from the constraint of how resources are distributed through hierarchical branching networks. Their understanding of an organism's metabolic/respiratory chain is based entirely on blood-flow considerations. Their claims have been repeatedly criticized as mistaken,[10] given that the role of fractal capillary branching is not demonstrated as fundamental to the exponent 3⁄4; and that blood-flow claims severely limit the relevance of the equation to organisms less than e−6 (≈ .0025) grams when the simultaneous claim is made that the equation is relevant over 27 orders of magnitude, extending from bacteria, which do not have hearts, to whales or forests.
Concepts of efficiency in the use of energy by the metabolism
This limit to blood flow considerations is problematic when claims are made that the theoretical models also are relevant to things without blood flow, like bacteria and coral. Attempts to understand the metabolic rate of a multi-cellular organism (field metabolic rate, that includes the activity of the organism) are couched in terms of the product between average basal metabolic rate, and number of cells.[11] This, plus capillary terminal size invariance, leaves the equation open to the criticism that it cannot possibly account for spikes in metabolic rate needed for motor activity. Too much blood would be required. This intimates that as proposed and popularly handled, the equation does not have the relevance to biology claimed, and is based upon assumptions that are not part of the equation, like fractality.
The WBE model can be modified to take into account metabolic efficiency, (ME). This term is a ratio of the efficiency of redox coupling between the biomass battery W, and the sources of chemical energy available to it, measured against loss to heat. ME is therefore a ratio of amperes of anabolism to amperes of catabolism.[12] When the term is added by hand to the exponent of the power law (which becomes (4ME-1)/4ME, where ME is metabolic efficiency), values for the exponent of 3⁄4 or 2⁄3, imply ME is 100 or 89% under this model. Efficiencies like these are not found in nature unless thermogenesis is included as part of metabolic rate. This removes the WBE version of Kleiber's law, which the metabolic theory of ecology rests on, from any biological relevance whatsoever. The efficiency that is purported to be modeled is actually assumed. In plants, according to a paper in 2006 in Nature, the exponent of mass is close to 1.[13][14] Under O'Kelly's model this is not possible since the implication is that ME is greater than 100% in the case of plants. The key problem is the nature of metabolic energy and the extent of what is considered metabolism. The problem is most clearly noticeable in the unit term for metabolic rate, i.e., calories/s. Calories are a measure of heat energy. This leads to the idea that thermogenesis is part of metabolism, Kleiber's original treatment, and rules out that metabolism is all about chemical energy, not heat energy. The picture is further obfuscated when the idea of respiratory metabolism is introduced to refine and limit the definition of metabolism such that oxygen consumption and synthesis of ATP are its ultimate factors. The data from study of oxygen consumption metabolic rates in cells in vitro suggests that the exponent is not only far less than 3⁄4, but also becomes negative for things less than one gram in size. Furthermore, glycogenesis is excluded from metabolic consideration on this model since glycogenesis is not included in the respiratory chain, and is itself a reduction reaction not strictly dependent upon the proximity of certain molecules and atoms delivered by capillaries and vibrating from Brownian motion. Energy is required for glycogenesis, and the blood does not deliver energy, just the ingredients for endergonic reactions. The energy comes from redox coupling, what ME is all about. Metabolic rate becomes the rate at which a biomass recharges so that its degeneration is prevented, and its organization is perpetuated. ME is here understood as a ratio of the rate of reduction reactions necessary for the maintenance, growth, replication and behavior of the biomass, to the rate of availability of energy captured and expended by that biomass. ME is a statement of redox coupling efficiency. ME excludes thermogenesis as part of metabolism, consequently. What influences BMR is the ME of the organism, not its mass. The organism determines ME, and that ME is the same for it and for its cells.
Other models taking into consideration how the energy is used, on the contrary, can accommodate thermogenesis as a part of the model. For example, a concept akin to the ME concept is the fraction f of the energy income that is not lost as heat.[6] This fraction is used for the metabolic work including the synthesis of proteins, cellular division, muscle contraction etc. being the remaining (1-f) the fraction lost as heat. Thus, as the ME, it depends on the energy stored and lost during the synthesis and use of ATP, and hence on redox coupling. Note that if f were 0, all the energy income would become heat, hence the organism should behave as a heater and metabolic scaling should be proportional to M2⁄3. On the contrary, if f were 1, all the energy income would be used in metabolic work, thus being the metabolic scaling directly proportional to the number of cells, i.e. to the mass. Real organisms are somewhere in between both ideal cases, therefore interpolating as f kM + (1-f) k'M2⁄3 where k and k' are Meeh prefactors. Thus, basal metabolism is no a pure power law anymore but the weighted sum of two. This evolutive tradeoff can explain several features of the metabolic scaling, as the curvature found in mammal data, or the better fit for low masses endotherms to 2⁄3 as an exponent.[6] f can be estimated through the difference between direct (heat generation) and indirect (oxygen consumption) calorimetry. Typical values for f are 15-20%.[15]
Current debate
Current discussion and debate in the literature has refrained from consideration of the theoretical foundations for Kleiber's law. The role of thermogenesis in metabolism remains unexplained, in part because Kleiber's law, as originally formulated, was based upon the idea that metabolic energy was entirely related to measurements of heat generation and loss (but see Ballesteros et al. for a recent counterexample). This appears in the unit of metabolic rate most favored, calories/s rather than watts (as in the version of Kleiber's law that includes ME, where ME is a ratio of redox amperes), and in the limitation of disputation as to whether ME is 89 or 100%. Others [e.g., Kozlowski and Konarzewski, John Speakman] have criticized West, Brown, and Enquist on the point that the size-invariance of capillaries, which is the same from leaves to mammalian blood flow, dooms attempts to account for motor activity as part of metabolism.[16][17] This is why metabolic rates are almost always associated with the organism at rest, where metabolic rate is figured by the basal rate for the cell rather than for the rate for the organism in its day-to-day life in the field. West et al. claim that Kleiber's law refers to the basal metabolic rate of an organism's cells, not the field metabolic rate of the organism itself, and regard field metabolic rate to be the product of the average basal metabolic rate and number of cells in the organism. Biologists point out that BMR cannot possibly account for motor activity even by this reckoning, and the equation is therefore of limited value either way.
That metabolic efficiency should deviate from the favored high values is not part of the current debate, even though it creates inconsistencies within standard models, especially with regard to the nature of aging and the nature of the metabolic relation between the cell and the organism's mass. The standard versions of the equation's exponent (those that do not consider ME) cannot account for the wide variation in the lifetimes between rodents and birds of similar mass. This inconsistency could be explained simply if rodents had an ME less than 25% whereas birds didn't. Nor can the standard exponent explain why primates live so long when mammals of far greater mass do not live correspondingly longer, e.g., humans vs. whales, or chimpanzees vs. buffaloes. This is a simple matter if ME's are 31% vs. 28% in the first case, and 30% vs. 27% in the second. The relation of cell metabolic rate to organism mass, a contentious subject for proponents of the standard exponent, is modeled as non-existent when ME is considered, appearing instead as the relation of the cell's metabolic rate to the organism's ME, where the ME of the organism is the same as for its cells.
Major proponents of the equation, in the form of 'quarter power scaling', always limit themselves to mass specific metabolic rates, where the mass is one gram. The equation shows that at one gram mass, metabolic rate is the same for all MEs. This is why one gram is favored. It eliminates the role of ME in the equation, and makes the exponent ¾ or ⅔ at least plausible to the initiate concerned with laboratory rather than mathematical study of metabolism. Gram specific masses limit the understanding of metabolism to the in vitro level, a limitation perpetuated by the unavailability of in vivo metabolic measuring equipment aside from oxygen-use and temperature monitoring. Attention to fundamental principles of the electrochemical nature and dependence of biomass, is deflected in favor of continuing disputation about the equation's relevance, the appropriateness of Euclidean considerations in a fractal world of capillary fluid dynamics, and the whispered depths to its secrets with regard to aging and to cancer, secrets unattainable so far. The inclusion of the term ME in the exponent allows for the energetic basis of biological organization to be modeled, where replication is biomass response to fluctuations in energy availability, and can be seen from bacterial multiplication and quorum sensing, to the relation between mating strategies and food supply in large mammals.
See also
References
- ↑ Max Kleiber (1932). "Body size and metabolism". Hilgardia. 6: 315–351. doi:10.3733/hilg.v06n11p315.
- ↑ Kleiber M (1947). "Body size and metabolic rate". Physiological Reviews. 27 (4): 511–541. PMID 20267758.
- ↑ Biographical sketch (with photo) of Max Kleiber Archived 2008-02-08 at the Wayback Machine.
- ↑ West, Geoffrey; Brown, James H.; Enquist, Brian J. (1997). "A General Model for the Origin of Allometric Scaling Laws in Biology". Science. 276 (5309): 122–6. doi:10.1126/science.276.5309.122. PMID 9082983.
- ↑ Shour, Robert (November 2012). "Entropy and its relationship to allometry". arXiv:0804.1924.
- 1 2 3 Ballesteros FJ, Martinez VJ, Luque B, Lacasa L, Valor E, Moya A (2018). "On the thermodynamic origin of metabolic scaling". Scientific Reports. 8: 1448:1–1448:10. Bibcode:2018NatSR...8.1448B. doi:10.1038/s41598-018-19853-6.
- ↑ Dodds, P. S.; Rothman, D. H.; Weitz, J. S. (2001). "Re-examination of the 3/4 law of metabolism". J. Theor. Biol. 209: 9–27. arXiv:physics/0007096. doi:10.1006/jtbi.2000.2238.
- ↑ Kolokotrones, T.; Savage, V.; Deeds, E. J.; Fontana, W. (2010). "Curvature in metabolic scaling". Nature. 464: 753–756. Bibcode:2010Natur.464..753K. doi:10.1038/nature08920.
- ↑ West GB, Brown JH, Enquist BJ (1997-04-04). "A general model for the origin of allometric scaling laws in biology". Science. 276 (5309): 122–6. doi:10.1126/science.276.5309.122. PMID 9082983.
- ↑ Banavar, J. R.; Moses, M. E.; Brown, J. H.; Damuth, J.; Rinaldo, A.; Sibly, R. M.; Maritan, A. (19 August 2010). "A general basis for quarter-power scaling in animals". Proceedings of the National Academy of Sciences. 107 (36): 15816–15820. Bibcode:2010PNAS..10715816B. doi:10.1073/pnas.1009974107. PMC 2936637. PMID 20724663.
- ↑ Savage VM, Allen AP, Brown JH, Gillooly JF, Herman AB, Woodruff WH, West GB (13 March 2007). "Scaling of number, size, and metabolic rate of cells with body size in mammals". Proc Natl Acad Sci U S A. 104 (11): 4718–23. Bibcode:2007PNAS..104.4718S. doi:10.1073/pnas.0611235104. PMC 1838666. PMID 17360590.
- ↑ OKelly GC (27 August 2009). "The terrestrial evolution of metabolism and life – by the numbers". Theor Biol Med Model. 6 (17): 1–8. doi:10.1186/1742-4682-6-17.
- ↑ Reich PB, Tjoelker MG, Machado JL, Oleksyn J (26 January 2006). "Universal scaling of respiratory metabolism, size, and nitrogen in plants". Nature. 439 (7075): 457–61. Bibcode:2006Natur.439..457R. doi:10.1038/nature04282. PMID 16437113.
- ↑ Metabolic Rate and Kleiber's Law
- ↑ Zotin AI (2010). "Thermodynamic Bases of Biological Processes". Cambridge Univ. Press.
- ↑ Kozlowski J, Konarzewski M (2004). "Is West, Brown and Enquist's model of allometric scaling mathematically correct and biologically relevant?". Functional Ecology. 18 (2): 283–9. doi:10.1111/j.0269-8463.2004.00830.x.
- ↑ Kozlowski J, Konarzewski M (2005). "West, Brown and Enquist's model of allometric scaling again: the same questions remain" (PDF). Functional Ecology. 19 (4): 739–743. doi:10.1111/j.1365-2435.2005.01021.x. Archived from the original (PDF) on 2011-07-20.
Further reading
- Rau AR (September 2002). "Biological scaling and physics". J. Biosci. 27 (5): 475–8. doi:10.1007/BF02705043. PMID 12381870.
- Wang Z, O'Connor TP, Heshka S, Heymsfield SB (November 2001). "The reconstruction of Kleiber's law at the organ-tissue level". J. Nutr. 131 (11): 2967–70. PMID 11694627.
- Whitfield, J. (2006). In the Beat of a Heart. Washington, D.C.: Joseph Henry Press.
External links
- Kleiber bio – UC Davis
- Of Mice and Elephants
- New Clues to Why Size Equals Destiny
- Woolley, Thomas. "3/4 and Kleiber's Law". Numberphile. Brady Haran.