Habitat fragmentation
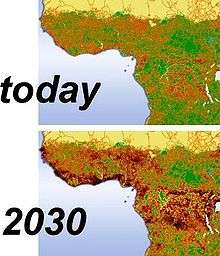
Habitat fragmentation describes the emergence of discontinuities (fragmentation) in an organism's preferred environment (habitat), causing population fragmentation and ecosystem decay. Causes of habitat fragmentation include geological processes that slowly alter the layout of the physical environment[1] (suspected of being one of the major causes of speciation[1]),and human activity such as land conversion, which can alter the environment much faster and causes the extinction of many species.
Definition
The term habitat fragmentation includes five discrete phenomena:
- Reduction in the total area of the habitat
- Decrease of the interior: edge ratio
- Isolation of one habitat fragment from other areas of habitat
- Breaking up of one patch of habitat into several smaller patches
- Decrease in the average size of each patch of habitat
"fragmentation ... not only causes loss of the amount of habitat, but by creating small, isolated patches it also changes the properties of the remaining habitat" (van den Berg et al. 2001). Habitat fragmentation is the landscape level of the phenomenon, and patch level process. Thus meaning, it covers; the patch areas, edge effects, and patch shape complexity.[2]
In scientific literature, there is some debate whether the term "habitat fragmentation" applies in cases of habitat loss, or whether the term primarily applies to the phenomenon of habitat being cut into smaller pieces without significant reduction in habitat area. Scientists who use the stricter definition of "habitat fragmentation" per se[3] would refer to loss of habitat area as "habitat loss" and explicitly mention both terms if describing a situation where the habitat becomes less connected and there is less overall habitat.
Causes
Natural causes
Evidence of habitat destruction through natural processes such as volcanism, fire, and climate change is found in the fossil record.[1] For example, habitat fragmentation of tropical rainforests in Euramerica 300 million years ago led to a great loss of amphibian diversity, but simultaneously the drier climate spurred on a burst of diversity among reptiles.[1]
Human causes
Habitat fragmentation is frequently caused by humans when native plants is cleared for human activities such as agriculture, rural development, urbanization and the creation of hydroelectric reservoirs. Habitats which were once continuous become divided into separate fragments. After intensive clearing, the separate fragments tend to be very small islands isolated from each other by cropland, pasture, pavement, or even barren land. The latter is often the result of slash and burn farming in tropical forests. In the wheat belt of central western New South Wales, Australia, 90% of the native vegetation has been cleared and over 99% of the tall grass prairie of North America has been cleared, resulting in extreme habitat fragmentation.
Endogenous vs. exogenous
There are two types of processes that can lead to habitat fragmentation. There are exogenous processes and endogenous processes. Endogenous are process that develop as a part of a species biology so they typically include changes in biology, behavior and interactions within or between species. Endogenous threats can result in changes to breeding patterns or migration patterns and are often triggered by exogenous processes. Exogenous processes are independent of species biology and can include habitat degradation, habitat subdivision or habitat isolation. These processes can have a substantial impact on endogenous processes by fundamentally altering species behavior. Habitat subdivision or isolation can lead to changes in dispersal or movement of species including changes to seasonal migration. These changes can lead to decrease in a density of species, increased competition or even increased predation.[4]
Implications
Habitat Loss and Biodiversity
One of the major ways that habitat fragmentation affects biodiversity is by reducing the amount of suitable habitat available for organisms. Habitat fragmentation often involves both habitat destruction and the subdivision of previously continuous habitat.[5] Plants and other sessile organisms are disproportionately affected by some types of habitat fragmentation because they cannot respond quickly to the altered spatial configuration of the habitat.[6]
Habitat loss, which can occur through the process of habitat fragmentation, is considered to be the greatest threat to species.[7] But, the effect of the configuration of habitat patches within the landscape, independent of the effect of the amount of habitat within the landscape (referred to as fragmentation per se[3]), has been suggested to be small.[8] A review of empirical studies found that, of the 381 reported significant effect of habitat fragmentation per se on species occurrences, abundances or diversity in the scientific literature, 76% were positive whereas 24% were negative.[9] Despite these results, the scientific literature tends to emphasize negative effects more than positive effects.[10] Positive effects of habitat fragmentation per se imply that several small patches of habitat can have higher conservation value than a single large patch of equivalent size.[9] Land sharing strategies could therefore have more positive impacts on species than land sparing strategies.[9]

Area is the primary determinant of the number of species in a fragment[11] and the relative contributions of demographic and genetic processes to the risk of global population extinction depend on habitat configuration, stochastic environmental variation and species features.[12] Minor fluctuations in climate, resources, or other factors that would be unremarkable and quickly corrected in large populations can be catastrophic in small, isolated populations. Thus fragmentation of habitat is an important cause of species extinction.[11] Population dynamics of subdivided populations tend to vary asynchronously. In an unfragmented landscape a declining population can be "rescued" by immigration from a nearby expanding population. In fragmented landscapes, the distance between fragments may prevent this from happening. Additionally, unoccupied fragments of habitat that are separated from a source of immigrants by some barrier are less likely to be repopulated than adjoining fragments. Even small species such as the Columbia spotted frog are reliant on the rescue effect. Studies showed 25% of juveniles travel a distance over 200m compared to 4% of adults. Of these, 95% remain in their new locale, demonstrating that this journey is necessary for survival.[13]
Additionally, habitat fragmentation leads to edge effects. Microclimatic changes in light, temperature and wind can alter the ecology around the fragment, and in the interior and exterior portions of the fragment. Fires become more likely in the area as humidity drops and temperature and wind levels rise. Exotic and pest species may establish themselves easily in such disturbed environments, and the proximity of domestic animals often upsets the natural ecology. Also, habitat along the edge of a fragment has a different climate and favours different species from the interior habitat. Small fragments are therefore unfavourable for species which require interior habitat. The percentage preservation of contiguous habitats is closely related to both genetic and species biodiversity preservation. Generally a 10% remnant contiguous habitat will result in a 50% biodiversity loss.[14]
Informed Conservation
Habitat fragmentation is often a cause of species becoming threatened or endangered. The existence of viable habitat is critical to the survival of any species, and in many cases the fragmentation of any remaining habitat can lead to difficult decisions for conservation biologists. Given a limited amount of resources available for conservation is it preferable to protect the existing isolated patches of habitat or to buy back land to get the largest possible continuous piece of land. In rare cases a conservation reliant species may gain some measure of disease protection by being distributed in isolated habitats. This ongoing debate is often referred to as SLOSS (Single Large or Several Small).
One solution to the problem of habitat fragmentation is to link the fragments by preserving or planting corridors of native vegetation. In some cases, a bridge or underpass may be enough to join two fragments.[15] This has the potential to mitigate the problem of isolation but not the loss of interior habitat.
Another mitigation measure is the enlargement of small remnants in order to increase the amount of interior habitat. This may be impractical since developed land is often more expensive and could require significant time and effort to restore.
The best solution is generally dependent on the particular species or ecosystem that is being considered. More mobile species, like most birds, do not need connected habitat while some smaller animals, like rodents, may be more exposed to predation in open land. These questions generally fall under the headings of metapopulations island biogeography.
Genetic Risks
As the remaining habitat patches are smaller, they tend to support smaller populations of fewer species.[16] Small populations are at an increased risk of a variety of genetic consequences that influence their long-term survival.[17] Remnant populations often contain only a subset of the genetic diversity found in the previously continuous habitat. Processes that act upon underlying genetic diversity such as adaptation have a smaller pool of fitness-maintaining alleles to survive in the face of environmental change.
Gene Flow and Inbreeding
Gene flow occurs when individuals of the same species exchange genetic information through reproduction. Populations can maintain genetic diversity through migration. When a habitat becomes fragmented and reduced in area, gene flow and migration is typically reduced. Fewer individuals will migrate into the remaining fragments, and small disconnected populations that may have once been part of a single large population will become reproductively isolated. Scientific evidence that gene flow is reduced due to fragmentation depends on the study species. While trees that have long-range pollination and dispersal mechanisms may not experience reduced gene flow following fragmentation,[18] most species are at risk of reduced gene flow following habitat fragmentation.[6]
Reduced gene flow, and reproductive isolation can result in inbreeding between related individuals. Inbreeding does not always result in negative fitness consequences, but when inbreeding is associated with fitness reduction it is called inbreeding depression. Inbreeding becomes of increasing concern as the level of homozygosity increases, facilitating the expression of deleterious alleles that reduce the fitness. Habitat fragmentation can lead to inbreeding depression for many species due to reduced gene flow.[19][20] Inbreeding depression is associated with conservation risks, like local extinction.
Genetic Drift
Small populations are more susceptible to genetic drift. Genetic drift is random changes to the genetic make up of populations and always leads to reductions in genetic diversity. The smaller the population is, the more likely genetic drift will be a driving force of evolution rather than natural selection. Because genetic drift is a random process, it does not allow species to become more adapted to their environment. Habitat fragmentation is associated with increases to genetic drift in small populations which can have negative consequences for the genetic diversity of the populations.[19]
Adaptation
In order for populations to evolve in response to natural selection, they must be large enough that natural selection is a stronger evolutionary force than genetic drift. Recent studies on the impacts of habitat fragmentation on adaptation in some plant species have suggested that organisms in fragmented landscapes may be able to adapt to fragmentation.[21][22] However, there are also many cases where fragmentation reduces adaptation capacity because of small population size.[23]
Examples of Impacted Species
Some species that have experienced genetic consequences due to habitat fragmentation are listed below:

Forest fragmentation
| Wikimedia Commons has media related to Forest fragmentation. |
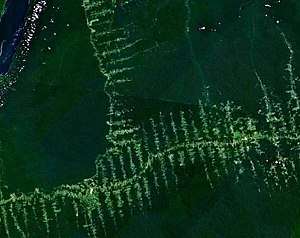
Forest fragmentation is a form of habitat fragmentation where forests are reduced (either naturally or man-made) to relatively small, isolated patches of forest known as forest fragments or forest remnants.[1] The intervening matrix that separates the remaining woodland patches can be natural open areas, farmland, or developed areas. Following the principles of island biogeography, remnant woodlands act like islands of forest in a sea of pastures, fields, subdivisions, shopping malls, etc. These fragments will then begin to undergo the process of ecosystem decay.
Forest fragmentation also includes less subtle forms of discontinuities such as utility right-of-ways (ROWs). Utility ROWs are of ecological interest because they have become pervasive in many forest communities, spanning areas as large as 5 million acres in the United States.[30] Utility ROWs include electricity transmission ROWs, gas pipeline and telecommunication ROWs. Electricity transmission ROWs are created to prevent vegetation interference with transmission lines. Some studies have shown that electricity transmission ROWs harbor more plant species than adjoining forest areas,[31] due to alterations in the microclimate in and around the corridor. Discontinuities in forest areas associated with utility right-of-ways can serve as biodiversity havens for native bees [30] and grassland species,[32] as the right-of-ways are preserved in an early successional stage.
Implications
Forest fragmentation is one of the greatest threats to biodiversity in forests, especially in the tropics.[33] The problem of habitat destruction that caused the fragmentation in the first place is compounded by:
- the inability of individual forest fragments to support viable populations, especially of large vertebrates
- the local extinction of species that do not have at least one fragment capable of supporting a viable population
- edge effects that alter the conditions of the outer areas of the fragment, greatly reducing the amount of true forest interior habitat.[34]
The effect of fragmentation on the flora and fauna of a forest patch depends on a) the size of the patch, and b) its degree of isolation. Isolation depends on the distance to the nearest similar patch, and the contrast with the surrounding areas. For example, if a cleared area is reforested or allowed to regenerate, the increasing structural diversity of the vegetation will lessen the isolation of the forest fragments. However, when formerly forested lands are converted permanently to pastures, agricultural fields, or human-inhabited developed areas, the remaining forest fragments, and the biota within them, are often highly isolated.
Forest patches that are smaller or more isolated will lose species faster than those that are larger or less isolated. A large number of small forest "islands" typically cannot support the same biodiversity that a single contiguous forest would hold, even if their combined area is much greater than the single forest. However, forest islands in rural landscapes greatly increase their biodiversity.[35]
Approaches to understanding habitat fragmentation
Two approaches that are typically used to understand habitat fragmentation and its ecological impacts.
Species-oriented approach
The species-oriented approach focuses specifically on individual species and how they each respond to their environment and habitat changes with in it. This approach can be limited because it does only focus on individual species and does not allow for a broad view of the impacts of habitat fragmentation across species.[36]
Pattern-oriented approach
The pattern-oriented approach is based on land cover and its patterning in correlation with species occurrences. One model of study for landscape patterning is the patch-matrix-corridor model developed by Richard Forman The pattern-oriented approach focuses on land cover defined by human means and activities. This model has stemmed from island biogeography and tries to infer causal relationships between the defined landscapes and the occurrence of species or groups of species within them. The approach has limitations in its collective assumptions across species or landscapes which may not account for variations amongst them.[37]
Variegation Model
The other model is the variegation model. Variegated landscapes retain much of their natural vegetation but are intermixed with gradients of modified habitat [38] This model of habitat fragmentation typically applies to landscapes that are modified by agriculture. In contrast to the fragmentation model that is denoted by isolated patches of habitat surrounded by unsuitable landscape environments, the variegation model applies to landscapes modified by agriculture where small patches of habitat remain near the remnant original habitat. In between these patches are a matrix of grassland that are often modified versions of the original habitat. These areas do not present as much of a barrier to native species.[39]
See also
Bibliography
- Lindenmayer D.B & Fischer J (2013) Habitat Fragmentation and Landscape Change: An Ecological and Conservation Synthesis (Island Press)
References
- 1 2 3 4 5 Sahney, S., Benton, M.J. & Falcon-Lang, H.J. (2010). "Rainforest collapse triggered Pennsylvanian tetrapod diversification in Euramerica" (PDF). Geology. 38 (12): 1079–1082. doi:10.1130/G31182.1.
- ↑ van den Berg LJL, Bullock JM, Clarke RT, Langsten RHW, Rose RJ. 2001. Territory selection by the Dartford warbler (Sylvia undata) in Dorset, England: the role of vegetation type, habitat fragmentation and population size. Biol. Conserv. 101:217-28
- 1 2 Fahrig, L (2003). "Effects of habitat fragmentation on biodiversity". Annual Review of Ecology, Evolution, and Systematics. 34: 487–515. doi:10.1146/annurev.ecolsys.34.011802.132419.
- ↑ Fischer, Joern; Lindenmayer, David B. (February 7, 2007). "Landscape Modification and Habitat Fragmentation: A synthesis". Global Ecology and Biogeography. 16 (3): 265–280. doi:10.1111/j.1466-8238.2007.00287.x. Retrieved March 22, 2018.
- ↑ Fahrig, Lenore (November 2003). "Effects of Habitat Fragmentation on Biodiversity". Annual Review of Ecology, Evolution, and Systematics. 34 (1): 487–515. doi:10.1146/annurev.ecolsys.34.011802.132419.
- 1 2 Lienert, Judit (July 2004). "Habitat fragmentation effects on fitness of plant populations – a review". Journal for Nature Conservation. 12 (1): 53–72. doi:10.1016/j.jnc.2003.07.002.
- ↑ Wilcove, David S.; et al. (1998). "Quantifying Threats to Imperiled Species in the United States". BioScience. 48 (8): 607–615. doi:10.2307/1313420. JSTOR 1313420.
- ↑ Fahrig, L (2013). "Rethinking patch size and isolation effects: the habitat amount hypothesis". J. Biogeogr. 40: 1649–1663. doi:10.1111/jbi.12130.
- 1 2 3 Fahrig, L (2017). "Ecological Responses to Habitat Fragmentation Per Se". Annual Review of Ecology, Evolution, and Systematics. 48: 1–23. doi:10.1146/annurev-ecolsys-110316-022612.
- ↑ Fahrig, L. (2018) Forty years of biais in habitat fragmentation research, In: Effective Conservation Science: Data Not Dogma (Edited by Kareiva, Marvier and Silliman), Oxford University Press, United Kingdom
- 1 2 Rosenzweig, Michael L. (1995). Species diversity in space and time. Cambridge: Cambridge University Press.
- ↑ Robert, A (2011). "Find the weakest link. A comparison between demographic, genetic and demo-genetic metapopulation extinction times". BMC Evolutionary Biology. 11: 260. doi:10.1186/1471-2148-11-260.
- ↑ Funk W.C.; Greene A.E.; Corn P.S.; Allendorf F.W. (2005). "High dispersal in a frog species suggests that it is vulnerable to habitat fragmentation". Biol. Lett. 1 (1): 13–6. doi:10.1098/rsbl.2004.0270.
- ↑ Quammen, David (1997), "The Song of the Dodo: Island Biogeography in an Age of Extinction" (Scribner)
- ↑ "Wildlife Crossings: Animals survive with bridges and tunnels". Wilder Eutopia. Retrieved 19 December 2017.
- ↑ Simberloff, Daniel (1 January 1998). "Small and Declining Populations". Conservation Science and Action. Blackwell Publishing Ltd.: 116–134. doi:10.1002/9781444313499.ch6.
- ↑ Frankham, Richard; Ballou, Jonathan D.; Briscoe, David A. (2009). Introduction to conservation genetics (2nd ed.). Cambridge: Cambridge University Press. ISBN 9780521702713.
- ↑ KRAMER, ANDREA T.; ISON, JENNIFER L.; ASHLEY, MARY V.; HOWE, HENRY F. (2008-06-09). "The Paradox of Forest Fragmentation Genetics". Conservation Biology. 22 (4): 878–885. doi:10.1111/j.1523-1739.2008.00944.x. ISSN 0888-8892.
- 1 2 Pavlova, Alexandra; Beheregaray, Luciano B.; Coleman, Rhys; Gilligan, Dean; Harrisson, Katherine A.; Ingram, Brett A.; Kearns, Joanne; Lamb, Annika M.; Lintermans, Mark (2017-05-11). "Severe consequences of habitat fragmentation on genetic diversity of an endangered Australian freshwater fish: A call for assisted gene flow". Evolutionary Applications. 10 (6): 531–550. doi:10.1111/eva.12484. ISSN 1752-4571. PMC 5469170. PMID 28616062.
- ↑ Wang, W; Qiao, Y; Li, S; Pan, W; Yao, M (2017-02-15). "Low genetic diversity and strong population structure shaped by anthropogenic habitat fragmentation in a critically endangered primate, Trachypithecus leucocephalus". Heredity. 118 (6): 542–553. doi:10.1038/hdy.2017.2.
- ↑ Matesanz, Silvia; Teso, Rubio; Luisa, María; García-Fernández, Alfredo; Escudero, Adrián (2017). "Habitat Fragmentation Differentially Affects Genetic Variation, Phenotypic Plasticity and Survival in Populations of a Gypsum Endemic". Frontiers in Plant Science. 8. doi:10.3389/fpls.2017.00843. ISSN 1664-462X.
- ↑ Dubois, Jonathan; Cheptou, Pierre-Olivier (2017-01-19). "Effects of fragmentation on plant adaptation to urban environments". Phil. Trans. R. Soc. B. 372 (1712): 20160038. doi:10.1098/rstb.2016.0038. ISSN 0962-8436. PMID 27920383.
- ↑ Legrand, Delphine; Cote, Julien; Fronhofer, Emanuel A.; Holt, Robert D.; Ronce, Ophélie; Schtickzelle, Nicolas; Travis, Justin M. J.; Clobert, Jean (2016-11-15). "Eco-evolutionary dynamics in fragmented landscapes". Ecography. 40 (1): 9–25. doi:10.1111/ecog.02537. ISSN 0906-7590.
- ↑ Pavlova, Alexandra; Beheregaray, Luciano B.; Coleman, Rhys; Gilligan, Dean; Harrisson, Katherine A.; Ingram, Brett A.; Kearns, Joanne; Lamb, Annika M.; Lintermans, Mark (2017-05-11). "Severe consequences of habitat fragmentation on genetic diversity of an endangered Australian freshwater fish: A call for assisted gene flow". Evolutionary Applications. 10 (6): 531–550. doi:10.1111/eva.12484. ISSN 1752-4571. PMC 5469170. PMID 28616062.
- ↑ "Macquaria australasica". fishesofaustralia.net.au. Retrieved 2018-06-06.
- ↑ Jump, Alistair S.; Peñuelas, Josep (2006-05-23). "Genetic effects of chronic habitat fragmentation in a wind-pollinated tree". Proceedings of the National Academy of Sciences. 103 (21): 8096–8100. doi:10.1073/pnas.0510127103. ISSN 0027-8424. PMID 16698935.
- ↑ "Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest". Biological Conservation. 142 (8): 1560–1569. 2009-08-01. doi:10.1016/j.biocon.2008.11.016. ISSN 0006-3207.
- ↑ Peacock, Mary M.; Smith, Andrew T. (1997-11-24). "The effect of habitat fragmentation on dispersal patterns, mating behavior, and genetic variation in a pika ( Ochotona princeps ) metapopulation". Oecologia. 112 (4): 524–533. doi:10.1007/s004420050341. ISSN 0029-8549.
- 1 2 3 4 Delaney, Kathleen Semple; Riley, Seth P. D.; Fisher, Robert N. (2010-09-16). "A Rapid, Strong, and Convergent Genetic Response to Urban Habitat Fragmentation in Four Divergent and Widespread Vertebrates". PLOS ONE. 5 (9): e12767. doi:10.1371/journal.pone.0012767. ISSN 1932-6203. PMC 2940822. PMID 20862274.
- 1 2 Russell, K. N.; Ikerd, H.; Droege, S. (2005-07-01). "The potential conservation value of unmowed powerline strips for native bees". Biological Conservation. 124 (1): 133–148. doi:10.1016/j.biocon.2005.01.022.
- ↑ Wagner, David L.; Metzler, Kenneth J.; Leicht-Young, Stacey A.; Motzkin, Glenn (2014-09-01). "Vegetation composition along a New England transmission line corridor and its implications for other trophic levels". Forest Ecology and Management. 327: 231–239. doi:10.1016/j.foreco.2014.04.026.
- ↑ Lampinen, Jussi; Ruokolainen, Kalle; Huhta, Ari-Pekka (2015-11-13). "Urban Power Line Corridors as Novel Habitats for Grassland and Alien Plant Species in South-Western Finland". PLOS ONE. 10 (11): e0142236. doi:10.1371/journal.pone.0142236. ISSN 1932-6203. PMC 4643934. PMID 26565700.
- ↑ Bierregaard, Richard (2001). Claude Gascon; Thomas E. Lovejoy; Rita Mesquita, eds. Lessons from Amazonia: The Ecology and Conservation of a Fragmented Forest. ISBN 0-300-08483-8.
- ↑ Harris, Larry D. (1984). The Fragmented Forest: Island Biogeography Theory and the Preservation of Biotic Diversity. The University of Chicago Press. ISBN 0-226-31763-3.
- ↑ Banaszak J. (ed.) 2000. Ecology of Forest Islands. Bydgoszcz University Press, Bydgoszcz, Poland, 313 pp.
- ↑ Fischer, Joern; Lindenmayer, David B. (February 7, 2007). "Landscape Modification and Habitat Fragmentation: A synthesis". Global Ecology and Biogeography. 16 (3): 265–280. doi:10.1111/j.1466-8238.2007.00287.x. Retrieved March 22, 2018.
- ↑ Fischer, Joern & B. Lindenmayer, David. (2007). Landscape modification and habitat fragmentation: a synthesis. Global Ecology and Biogeography. 16. 265-280. 10.1111/j.1466-8238.2007.00287.
- ↑ "Landscape Ecology and Landscape Change" (PDF). Retrieved March 22, 2018.
- ↑ McIntyre, S., and G. W. Barrett. “Habitat Variegation, An Alternative to Fragmentation.” Conservation Biology, vol. 6, no. 1, 1992, pp. 146–147. JSTOR, JSTOR, www.jstor.org/stable/2385863.
External links
| Wikimedia Commons has media related to Ecological fragmentation. |
- GLOBIO, an ongoing programme to map the past, current and future impacts of human activities on the natural environment, specifically highlighting larger wilderness areas and their fragmentation
- Monash Virtual Laboratory – Simulations of habitat fragmentation and population genetics online at Monash University's Virtual Laboratory.
- Defragmentation in Belgium (Flanders) – Connecting nature, connecting people. Accessed: Jan 22, 2009
- Wildlife passages – De-Fragmentation in the Netherlands – How to evaluate their effectiveness? Accessed: Jan 22, 2009
- Landscape Fragmentation in Europe The technical report from 2006 - the result of a collaboration between the Swiss Federal Office for the Environment (FOEN) and the European Environment Agency (EEA). Accessed: Feb 22, 2016
- Kinver, Mark. (2013, September 26). "Forest fragmentation triggers 'ecological Armageddon'", BBC News.