Elasmotherium
| Elasmotherium | |
|---|---|
| Reconstructed E. caucasicum skeleton, Asowschen historische und archäologische und paläontologische Museum-Reserve | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Infraclass: | Eutheria |
| Order: | Perissodactyla |
| Suborder: | Ceratomorpha |
| Superfamily: | Rhinocerotoidea |
| Family: | Rhinocerotidae |
| Subfamily: | Elasmotheriinae |
| Tribe: | Elasmotheriini |
| Genus: | †Elasmotherium J. Fischer, 1808[1] |
| Species | |
| |
Elasmotherium ("Thin Plate Beast") is an extinct genus of rhinoceros endemic to Eurasia during the Late Pliocene through the Pleistocene, documented from 2.6 Ma to as late as 29,000 years ago in the Late Pleistocene.[2][3] Three species are recognised. The best known, E. sibiricum, was the size of a mammoth and is thought to have borne a large, thick horn on its forehead. Theories about the function of this horn include defence, attracting mates, driving away competitors, sweeping snow from the grass in winter and digging for water and plant roots. Like all rhinoceroses, elasmotheres were herbivorous. Unlike any others, its high-crowned molars were ever-growing. Its legs were longer than those of other rhinos and were adapted for galloping, giving it a horse-like gait.
Taxonomy
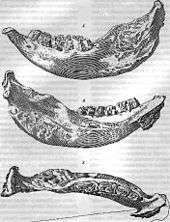
The fossil received its name from Johann Fischer von Waldheim,[4] the Dirécteur Perpétuel of the Natural History Museum, Moscow University, at a presentation before the Societé Impériale des Naturalistes in 1808. The next year in the Mémoires of the society he reported what he had said in the Programme d'invitation:[5]
Elasmotherium is an animal with an elongated head without incisors or canines, with 5 molars on each side [made] from sinuous layers.[6]
Then he noted on his derivation of the name:
From the Greek word ἐλασμος [elasmos], layer, to designate the layered form of the molar teeth.[7]
All he had before him was one lower jaw donated to the museum by Yekaterina Romanovna Vorontsova-Dashkova, which he named Elasmotherium sibiricum, lamenting that it was the sole species of which he knew. The molars, the only teeth in the jaw, had formed in layers like tree rings, except the "rings", or lamellae, were highly corrugated. The edges in the grinding surface were elaborately sinuous to better break down the grasses on which the animal fed.
In March 2016, the discovery of a skull in Kazakhstan granted a new estimated time period to when Elasmotherium roamed the Earth. The prior estimate was 350,000 years ago, now being reduced to 29,000 years ago.[8][9]
The genus is known from hundreds of find sites, mainly of cranial fragments and teeth, but in some cases nearly complete skeletons of post-cranial bones, scattered over Eurasia from Eastern Europe to China. For example, Kazakhstan alone has 30 sites of E. sibiricum.[10] Dozens of crania have been reconstructed and given archaeological identifiers. The division into species is based mainly on the fine distinctions of the teeth and jaws and the shape of the skull.[11] The finds can be dated only by context.
Species

Elasmotherium is believed to have descended from the Late Pliocene genus of Central Asia, Sinotherium. Elasmotherium is thought to be the most derived genus of elasmothere, with E. caucasicum in turn being more derived than E. sibiricum.[12] The two Chinese fossils, formerly considered distinct species, E. inexpectatum and E. peii, defined by Chow in 1958, have been sunk into E. caucasicum.[13] They were found in northern China from the Early Pleistocene Nihewan Faunal assemblage (from the same valley as nearby Nihewan in Shanxi) and were extinct at approximately 1.6 Ma.[14]
E. caucasicum, defined by Borissjak in 1914, flourished in the Black Sea region, as a member of the Early Pleistocene Tamanian Faunal Unit (1.1–0.8 Ma, Taman Peninsula). However, an elasmotherian species turned up in the preceding Khaprovian or Khaprov Faunal Complex, which was at first taken to be E. caucasicum,[15] and then on the basis of the dentition was redefined as a new species, E. chaprovicum (Shvyreva, 2004), named after the Khaprov Faunal Complex.[11] The Khaprov is in the MIddle Villafranchian, MN17, which spans the Piacenzian of the Late Pliocene and the Gelasian of the Early Pleistocene of Northern Caucasus, Moldova and Asia and has been dated to 2.6–2.2 Ma.[16]
E. sibiricum, described by Johann Fischer von Waldheim in 1808 and chronologically the latest species of the sequence, coming from E. caucasicum in the Middle Pleistocene, ranged from southwestern Russia to western Siberia and southward into Ukraine and Moldova. It appears in the Middle Pleistocene Khazar Faunal Complex of the Sea of Azov region, which has "no exact stratigraphic situation".[17]
The end of the elasmotheres is questionable; new evidence continues to turn up. The latest is from two caves in southern Siberia, a E. sibericum tooth in Smelovskaya Cave 11[18] and remains from Batpak,[19] both associated with Middle Pleistocene relict species, including large herbivores and predators. They must have been dragged into the caves by some predator, perhaps even modern man. The elasmothere tooth and one of a cave hyena from Smelovskaya were carbon dated to slightly greater than 50,000 BP in the Late Pleistocene.[20] A recent study of relict Late Pleistocene remains in the Beringia region (Alaska, Eastern Siberia) identified a pattern of "imbedded micrometeorites" consistent with a space impact event similar to the Tunguska event of 1908, only carbon-dated to 37,000 BP. A Siberian Elasmotherium skull in a museum was found to have this pattern.[21]
Evolution
The evolution of Rhinocerotidae follows a very similar pattern to the evolution of the Equidae, both being facets of the evolution of mammalian herbivores during the Cenozoic. The overall pattern is based on the evolutionary development of grass and grassland, and further the changing distribution of C3 and C4 types of plant metabolism, so-called because they generate a molecule containing three or four Carbon atoms by different metabolic pathways (sequences of chemical reactions).
The rhinocerotoids of the early Eocene and subsequently the Hyracodontidae and Amynodontidae show no sign of dermal armour or horns. The oldest known genus of rhinocerotids, or true rhinoceroses, is Teletaceras from the Middle Eocene of North America and the Late Eocene of Asia. Due to the timing of land bridges, it does not appear in South America.[22] It features the first known rhinocerotid tusks, a derived feature, which are still extant in three species and are "the primary offensive weapons" of those species. They appear to have been sexually dimorphic features convergent with the development of tusks in pigs, hippopotamuses and elephant seals.[23]
The first dermal armour is indicated by cranial rugosity on Trigonias osborni, Late Eocene, 42-32 Mya. It appears more developed in Subhyracodon, early Oligocene, 33 Mya, both of North America.[24] There appears to have been a correlation between the tusks and the armour later (4 Mya later) defending against them.[25]
Having begun to diversify (increase in species and genera) in America the rhinocerotids spread rapidly over Beringia when that bridge appeared in the Early Oligocene to Eurasia, where subsequently they became highly diverse. The family reached a maximum floruit in the Miocene. At that time, they entered Africa, but never were very diverse there. In the Pliocene they declined, disappearing from America. The Pleistocene brought extinction from Eurasia, but African and Southeast Asian species survived.[22]
Elasmotherium appeared in the Late Pliocene, apparently deriving from the Miocene—Pliocene Sinotherium, sometimes informally aggregated with it. Sinotherium in turn appeared to be related to Iranotherium; that is, Elasmotherium was believed to have been an iranotheriine. A 1995 cladistic analysis by Cerdeño asserted that Elasmotherium was most closely related to Coelodonta, the "woolly rhinoceros", whose soft-tissue corpus has been found in a few locations. By implication, Sinotherium was not an iranothere.[26] However, Antoine's 2002 analysis reaffirmed that the elasmotheriine line was iranotheriine. Certainty concerning the exact line of descent remains elusive; fossils and techniques of the future will perhaps resolve it.
The evolution of Rhinocerotidae in the Miocene grasslands began when diminutive, cursorial, browsing, brachydont species expanded their ranges from the Oligocene forests into Miocene grasslands and there, over the millions of years, evolved diverse and flourishing populations, gradually increasing in size.[27] Some of these species exploited the grazing niche. Hispanotherium, Chilotherium and Teleoceras "show a clear tendency to hypsodonty, and a grazing feeding adaptation has usually been attributed to them".
By the late Miocene, grasslands changed to the savanna, today's subtropical mixture of grasses and sedges. Smaller, browsing Rhinocerotidae declined in diversity, while grazers gained in size and weight.[28] Massive grazers, such as Elasmotherium, maximally hypsodont, moved across vast ranges consuming grass in bulk. While horses and other more nearly modern grazers could rely on galloping for mobility in any circumstance, the horns and impenetrable body armour insured that the giants would not be required to gallop away from any predator.
The classification of all the species and genera of the Rhinoceros family below the level of Rhinoceratidae has changed more rapidly in the first decade of the 21st century due to first the continued discovery of fossils representing new species and genera from China and Mongolia and second the number of points of morphology to be compared in computerised phylogenetic analysis. Two branches are generally recognised, that leading to the smaller Rhinoceroses and the Pliocene/Pleistocene branch of the larger elasmotheres. Both began from small species in the steppes of the Far East, but those in turn are comparable to North American species. Whether the branch point is considered to be at the subfamily level (Elasmotheriinae versus Rhinocerotinae) or at the tribe level (Elasmotheriini versus Rhinocerotini, the currently accepted version) or whether the elasmotheres existed at the subtribe level (Elasmotheriina, a discontinued taxon) is a matter of the status quo in publications, fossils and software programs.
In 2002 P. O. Antoine performed a cladistic analysis using 282 "cranial, dental and postcranial characters"[29] of 28 "terminal taxa"[30] of "elasmotheriines from China and Mongolia" and four outgroups. He found that the elasmotheres were a monophyletic group. He says:
The main characters of derived elasmotheres (huge size, frontal horn, ossified nasal septum, enamel folding, hypsodonty, loss of anterior dentition, lengthening of the molar series ...) are absent in Middle Miocene Elasmotheriina from China and Mongolia. Most of these features appear somewhat later, during the Late Miocene or the Pliocene, in Parelasmotherium, Sinotherium and then Elasmotherium.
Antoine was following the now discontinued practice of considering the Miocene ancestral species as elasmotheres. They were not, however, distinguished by the features of an elasmothere. The original ancestors were "minute brachydont animals". Elasmotherium was a "mammoth-sized hypsodont". In his view,
Such cranio-dental evolution demonstrates unequivocally the increasing proportion of grass-eating in the elasmotherine diet throughout the Middle Miocene.
V.A. Terjaev in 1948 had a different view of the cause of the hypsodonty, maintaining that it was caused by the heavier grains in soil on plants pulled from moister environments, and that consequently Elasmotherians lived in "riparian biotopes".[14] Noskova points out that Elasmotherium's diet was comparable to that of the concurrent Archidiskodon, which ranged over both steppe and riverland.
Description
Various theories of Elasmothere morphology, nutrition and habits have been the cause of wide variation in reconstruction. Some show the beast trotting like a horse with a horn; others hunched over with head to the ground, like a bison, and still others immersed in swamps like a hippopotamus. The use of the horn and whether or not there was one, and how large, have been popular topics. The statistical correlations of modern palaeontology have taken much of the speculation out of the subject, although some details remain undetermined.
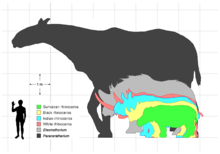
The known specimens of E. sibiricum reach up to 4.5 m (15 ft) in body length with shoulder heights over 2 m (6 ft 7 in) while E. caucasicum reaches at least 5 m (16 ft) in body length with an estimated mass of 3.6–4.5 tonnes (4–5 short tons), based on isolated molars that significantly exceed those known from the Siberian species.[31] Both species were among the largest in the family Rhinocerotidae, comparable in size to the woolly mammoth and larger than the contemporary woolly rhinoceros.[32][33] The feet were unguligrade, the front larger than the rear, with 3 digits at the front and rear, with a rudimentary fifth metacarpal.[34]
Horn morphology
The Ceratomorpha are so-called because their families, such as the Rhinocerotidae, of which Elasmotherium is indisputably one, are characterised by the presence of hooves, or horns and hooves, made of keratin, the same substance of which hair is made. These keratin structures appear to have formed in the Mesozoic, a remnant in humans being the nails. A keratin horn is to be distinguished from a bone horn and a tusk. Bone forms the base of most horns, but in some cases the horn is entirely of bone. A tusk is a modified canine or incisor tooth. Rhinocerotidae have had tusks, but not Elasmotherium. Two open questions are whether they were horned or hornless, hairy or hairless. Most Rhinocerotidae have and have had horns, but there are some instances of hornlessness, and most are or were hairy, such as the woolly rhinoceros, but no instances of hair or horn have yet been found for Elasmotherium. Only circumstantial evidence of them exists.
The main evidence suggestive of a horn on Elasmotherium is a frontal protuberance, which struck the attention of the late 19th century palaeontologists and was immediately interpreted as the bony basis for a horn by most investigators from that time forward. A skull of E. sibiricum from the Volga region (cast shown in this article's lead picture) described by Alexander Brandt in the Russian journal, Niwa, and reported in Nature in 1878 offers the following description of the protuberance: hemispherical, 5 inches (13 cm) deep, furrowed surface, circumference of 3 feet (0.91 m). The furrows are interpreted as the seats of blood vessels for the tissues that generated the horn:[35]
The whole analogy with the rhinoceros points with the greatest certainty to the previous existence of a horn, which, to judge from the size of the blood vessels once encircling the base, must have possessed enormous dimensions.
Brandt was already familiar with the legend of a unicorn among the Tatars of Siberia with a horn so large it required a sledge for transport, and made the connection in interpreting the bump as the base of a horn. He also interpreted the rostrum of the upper mandible as the basis of a nasal horn, a hypothesis now rejected in favour of the cropping labia. In any case, the non-circular base indicates a section through the horn would not have been circular. This possibility is supported by another fossil with a non-circular partially healed puncture wound in the base, chiefly interpreted as the result of dueling other males with the horn.[36]
The ungulates typically combine keratin and bone in various structures. If horns are keratinous, they have a bone core. Rhinocerotids horns, however, are uniquely derived. Hieronymus, an expert in Rhinoceros dermatology, says:[37]
... extant rhinocerotids are unique in possessing a massive entirely keratinous horn that approximates the functions of keratin-and-bone horns such as those of bovid artiodactyls ...."
He defines rhinocerotid horns as:
... cylindrical blocks of constantly growing cornified papillary epidermis.

This tissue is "strikingly convergent" with other "cornified epidermis" in horses, cetaceans, artiodactyls and birds. The horn is not attached to the bone of the boss but grows from the surface of a dense dermal tissue. The top layer keratinises itself to form tubules about 1–2 millimeter high, the cells of which then die. The next layer forms below it.[38] As the layers age the horn loses diameter by degradation of the keratin due to ultraviolet light, desiccation and mechanical wear from contact with objects and agonistic behaviour.[39] However, melanin and calcium deposits in the centre harden the keratin there, causing differential wear and shaping of the horn.[40]
The dermis generating the horn is anchored to the boss by interpenetration between rugosities – various irregularities of bone, which it creates by deposition.[41] This tissue is a specialisation of dermal armour, which, whenever it attaches to bone, deposits the rugosities to strengthen the attachment. The author states that rugosity is "a bony signature of dermal armor".[42] Cranial rugosity is an indication, but not a sure sign, of a horn. If, on the other hand, an annular (ring-shaped) pattern is visible in the rugosity, it is due to "stress concentration at the edges of horns" and is "the signature for epidermal horns".
Hieronymus found annular rugosities in all living and some fossil Rhinocerotidae. The rings had previously been noted on additional fossils. To date Elasmotherium has not been examined for rings under lighting designed to show them up; however, based on the observations of other palaeontologists, the author says "squamosal rugosity" is the "most pronounced cranial rugosity in the elasmotherine lineage".[43] This fact suggests an especially firm attachment was required, which, combined with the extraordinarily large hump of muscle for managing the head, could suggest a large and heavy horn. In the early 19th century the state of the fossils had not yet revealed the presence of a horn. From around 1910 and ever since then, palaeontologists have not ventured a mathematical estimate but rather have preferred occasionally to refer to the horn as immense, enormous, great or huge (these words turn up more often in popularising works than in scientific articles). As the size and shape of the horn would depend on the concentration of melanin and calcium, and no known indicator of those remains, any further estimate of horn morphology is purely speculative.
Dentition
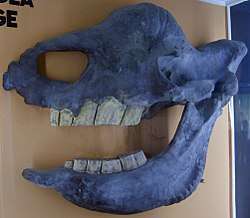
A third type of evidence that Elasmotherium was a grazer is that of tooth wear and morphology. Like all Rhinocerotidae, E. had cheek teeth evolved for herbivory: two premolars and three molars (originally taken for five molars), no incisors, no canines.[44] Where some of the browsers kept the incisors in the form of tusks, E, had instead a spoon-like symphysis, or tip, of the lower mandible and a rostrum, or beak, of the upper, which served as a bony basis for a soft-tissue labial grasping and tearing mechanism.[36] Grass, a very tough, fibrous material, contains phytoliths, microscopic granules mainly of silica, which act as sandpaper on the molars of grazers. Their response in geologic time is to evolve cheek teeth with large crowns (hypsodonty). There appears to be a correlation between grazing and hypsodonty:[45]
As a general rule, extant herbivores with low-crowned teeth are predominantly browsers and species with high-crowned teeth are predominantly grazers.
Vladimir Onufryevich Kovalevsky first proposed a connection between hypsodonty and grazing for horses in 1873.[46] Since then the concept has been expanded to all mammalian grazers at any time and has further been elaborated into hypsodonty or proto-hypsodonty and hypselodonty or euhypsodonty.[47] The euhypsodonts, of which, surprisingly for its bulk, E. was one,[48] have ever-growing high-crowned teeth. Most other examples are to be found among diminutive mammals such as Rodentia, which already casts doubt on the correlation, as they do not generally graze grass.
Teeth form from the top down through the deposition of enamel on a cement core by formative soft tissue in the jaw. The enamel of hypsodont Perissodactyla is highly rugose rather than sharp. In brachydont species, such as humans, when the crown is complete, the roots are deposited and finally the completed tooth erupts. Hypselodonty is a condition of tooth eruption and continued crown formation before a delayed root formation. In its most developed variety, the roots never form. Only rare fossils of E. show any sign of a root, and that on a premolar. No molars have roots, or, in the terminology of some, the roots are "open".
Habitat


If all grazers are hypsodont, not all hypsodonts are grazers. The supposed correlation between grass-eating and hypsodonty proved difficult to support in a number of instances. Koenigswald, for example, pointed out that hypsodonty had occurred among the Gondwanatheria of the Mesozoic, a group of mammals so primitive that he describes their cheek teeth as "molariform", as they are neither clearly molars nor premolars.[50] Molariform hypsodonty "cannot be correlated with a grass diet, since grasses were not present". Instead he suggests for his example, Sudamerica ameghinioi, that it lived a "semiaquatic and perhaps a burrowing way of life". Modern species provide many examples, from beavers to hippopotamuses.
Attempts have been made to link the wear on Elasmotherium teeth to grazing. In 1938, H.E. Wood, a Rhinocerotid tooth specialist, pointed out that interproximal wear, or loss of tooth surface between teeth, due to abrasion during mastication, of Elasmotherium is similar to that of the white rhinoceros, the only remaining Rhinocerotid grazer, which has hypsodont teeth.[51] Data such as this led to an intuitive concept of some sort of correlation between grass-eating and hypsodonty, but it has been difficult to isolate mathematically.
A 2007 study by Mendoza and Palmqvist compared the habitats and diets of 134 species of living ungulates, for which this data is known, both Artiodactyla and Perissodactyla, against "32 craniodental measurements" to discover what correlations exist for the group studied and to test hypotheses concerning hypsodonty and mode of life.[52] Habitats considered were open (savanna, deserts), mixed (wooded savanna, brush) and closed (riverine and forest). Diets considered were grazing, mixed grazing and browsing, browsing, omnivory and special niches, such as treetop browsing. Measurements included the Hypsodonty Index (HI) and Muzzle Width (MZW). The results showed that, except for the "high-level" browsers, hypsodonty is correlated to "open and mixed habitats". The HI was not precise enough to discriminate between open and mixed. However, high MZW is correlated to grazing in the open category, although some forest species also have wide muzzles. Grazers therefore are distinguished by a combination of high-crowned cheek teeth and wide muzzles, both of which are possessed by Elasmotherium. Life in the open is implied.
Palaeobiology
Diet
Herbivores can be divided into two general groups on the basis of nutrition, which grade into each other morphologically: "foregut fermenters" and "hindgut fermenters". The border region is correlated to bulk: up to 600 to 1,200 kilograms (1,300 to 2,600 lb) are the former; over it, the latter. In foregut fermentation, the animal must "browse" to select the most nutritious plants and then ruminate to make up for the shorter digestive tract. The hind-gut fermenters are "bulk-feeders": they ingest large quantities of low-nutrient food, which they process for a longer time in a much longer intestine. The main food in that category is grass, indicating that Elasmotherium, like the elephants, was probably a grassland "grazer" moving over long distances to take advantage of the growth phases of grass in different regions.[53] The standard is not without exception, as Indricotherium, the largest land mammal ever, with a weight of 15–20 tons, subsisted by browsing the treetops.
There are also palaeontological indications of the grazing. In general, the normal position of the head can be determined by the angle between a vertical plane coinciding with the occiput of the cranium, which is always vertical, and a plane through the base of the cranium. A right or acute angle would indicate a head held high for browsing leaves at various heights. Elasmotherium had the most obtuse angle of the Rhinocerotids. It could only reach the lowest levels and therefore must have grazed habitually.[54] This morphological feature favours the identification of the one-horned beast depicted in Rouffignac Cave (shown in this article) as Elasmotherium and lends some validity to the bison-like restorations based on it.
Cursorial distal limbs
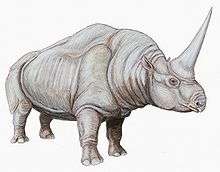
The preponderance of evidence is that Elasmotherium, as far as is known now, was a grassland grazer. It is in this context that Deng and Zheng, experts in the few surviving leg bones, conclude, concerning the morphology of the legs:[55]
Combined with hypsodont cheek teeth with much cement and strong enamel plication, the slender distal limb bones of E. caucasicum indicate that it is cursorial and dwells in an open steppe as a typical grazer.
By distal they mean "furthest outward"; that is, the extremities. Cursorial animals are unequivocally "runners" although the authors did not examine what sort of gait scientifically should be proposed as "running". They selected caucasicum for study because of the availability of a few dozen limb bone fragments from Nihewan, China. These made possible a selective comparison with the fewer bones remaining of other fossil rhinocerotids. In comparison with them, the long legs of Elasmotherium. are the most derived; that is, the others did not have the same cursorial capabilities. The authors approach but do not solve the problem of how to reconcile the weight with the supposed mobility. They say elsewhere in the article that the legs of caucasicum are to be distinguished from those of other fossil Rhinocerotids at Nihewan by their "enormous size".[56]
The white rhinoceros at an estimated weight of 2.3 tonnes[57] has been photographed galloping at a speed of about 30 km/h (19 mph). In a gallop, all feet are off the ground ("ballistic phase") twice a cycle, a feat that elephants, at 2.7–10 tonnes,[58][59] cannot perform. They can walk up to 20 km/h (12 mph); however, their straight, relatively inflexible legs are those of striders, not the bent and spring-like legs of gallopers, which use haunches, ankle mobility and knee flexion to spring off the ground on alternative legs of a pair. Elasmotherium legs are sufficiently like those of the White Rhino to hypothesize a similar gait even though Elasmotherium weighed 4.5-5 ton.[60]
See also
Notes
- ↑ "Elasmotherium". PaleoBiology Database: Basic info. National Center for Ecological Analysis and Synthesis. 2000. Retrieved 23 March 2011.
- ↑ Brait, Ellen (29 March 2016). "Extinct 'Siberian unicorn' may have lived alongside humans, fossil suggests". The Guardian.
- ↑ Shpansky, A. V.; Aliyassova, V. N.; Ilyina, S. A. (15 February 2016). "The Quaternary Mammals from Kozhamzhar Locality (Pavlodar Region, Kazakhstan)". American Journal of Applied Sciences. 13 (2): 189–199. doi:10.3844/ajassp.2016.189.199. Retrieved 31 March 2016.
- ↑ J. Fischer, who signed G. Fischer from a middle name, Gotthelf.
- ↑ Fischer, G. (1809). "Memoires de la Société Impériale des Naturalistes de Moscou". Tome II. Moscou: Imprimerie de l'Université Impériale: 255 (21. Sur L'Elasmotherium et le Trogontothérium). .
- ↑ "L'elasmotherium est un animal à tête elongée sans dents incisives et sans canines, de chaque coté 5 molaires à lames contournées."
- ↑ "Du mot grec ἐλασμος, lamelle, pour designer la forme lamelleuse des dents molaires."
- ↑ Dicker, Rachel (28 March 2016). "Unicorns Were Real, and a New Fossil Shows When They Lived". U.S. News. Kazakhstan. Retrieved 28 March 2016.
- ↑ HRALA, JOSH (27 March 2016). "A fossilised skull has revealed when the last 'Siberian unicorn' lived on Earth". ScienceAlert. Kazakhstan. Retrieved 28 March 2016.
- ↑ Tleuberdina, Piruza; Nazymbetova, Gulzhan (2010). "Distribution of Elasmotherium in Kazakhstan". In Titov, V.V.; Tesakov, A.S. Quaternary stratigraphy and paleontology of the Southern Russia: connections between Europe, Africa and Asia: Abstracts of the International INQUA-SEQS Conference (Rostov-on-Don, June 21–26, 2010) (PDF). Rostov-on-Don: Russian Academy of Science. pp. 171–173
- 1 2 Titov 2008, p. 226
- ↑ Deng & Zheng 2005, p. 119
- ↑ Deng & Zheng 2005, p. 112
- 1 2 Noskova, N.G. (2001). "Elasmotherians – evolution, distribution and ecology". In Cavarretta, G.; Gioia, P.; Mussi, M.; et al. The World of Elephants (PDF). Roma: Consiglio Nazionale delle Ricerche. pp. 126–128. ISBN 88-8080-025-6
- ↑ Logvynenko, Vitaliy. "The Development of the Late Pleistocene to Early Middle Pleistocene Large Mammal Fauna of Ukraine" (PDF). 18th International Senckenberg Conference 2004 in Weimar (Abstracts). Senckenberg Gesellschaft für Naturforschung (SGN).
- ↑ Bajgusheva, Vera S.; Titov, Vadim V. "Results of the Khapry Faunal Unit revision" (PDF). 18th International Senckenberg Conference 2004 in Weimar (Abstracts). Senckenberg Gesellschaft für Naturforschung (SGN).
- ↑ Baigusheva, Vera; Titov, Vadim (2010). "Pleistocene Large Mammal Associations of the Sea of Azov and Adjacent Regions". In Titov, V.V.; Tesakov, A.S. Quaternary stratigraphy and paleontology of the Southern Russia: connections between Europe, Africa and Asia: Abstracts of the International INQUA-SEQS Conference (Rostov-on-Don, June 21–26, 2010) (PDF). Rostov-on-Don: Russian Academy of Science. pp. 24–27
- ↑ 52°27′N 59°30′E / 52.450°N 59.500°E
- ↑ 50°30′N 72°30′E / 50.500°N 72.500°E
- ↑ Kosintsev, Pavel (2010). "Relict Mammal Species of the Middle Pleistocene in Late Pleistocene Fauna of the Western Siberia South". In Titov, V.V.; Tesakov, A.S. Quaternary stratigraphy and paleontology of the Southern Russia: connections between Europe, Africa and Asia: Abstracts of the International INQUA-SEQS Conference (Rostov-on-Don, June 21–26, 2010) (PDF). Rostov-on-Don: Russian Academy of Science. pp. 78–79
- ↑ Hagstrum, J. T.; Firestone, R. B.; West, A. (2009). "Beringian Megafaunal Extinctions at ~37 ka B.P.: Do Micrometeorites Embedded in Fossil Tusks and Skulls Indicate an Extraterrestrial Precursor to the Younger Dryas Event?". American Geophysical Union, Fall Meeting 2009, abstract #PP31D-1385. The Smithsonian/NASA Astrophysics Data System. 31: 1385. Bibcode:2009AGUFMPP31D1385H.
- 1 2 Cerdeño 1998, pp. 18–19
- ↑ Hieronymus 2009, p. 37.
- ↑ Hieronymus 2009, pp. 34–35
- ↑ Hieronymus 2009, p. 37
- ↑ Cerdeño 1998, p. 23
- ↑ Cerdeño 1998, p. 29
- ↑ Cerdeño 1998, pp. 28–29
- ↑ Antoine 2003, p. 109
- ↑ Antoine 2003, p. 107
- ↑ Zhegallo 2005, pp. 20–21
- ↑ Cerdeño, Esperanza; Nieto, Manuel (1995). "Changes in Western European Rhinocerotidae related to climatic variations" (PDF). Palaeo (114): 328.
- ↑ Cerdeño 1998, p. 25
- ↑ Belyaeva, E.I. (1977). "About the Hyroideum, Sternum and Metacarpale V Bones of Elasmotherium sibiricum Fischer (Rhinocerotidae)" (PDF). Journal of the Palaeontological Society of India. 20: 10–15.
- ↑ Brandt, Alexander; Lockyer, Norman (8 August 1878). "The Elasmotherium". Nature. XVIII: 287–389. Bibcode:1878Natur..18R.387.. doi:10.1038/018387b0.
- 1 2 Zhegallo 2005, p. 21
- ↑ Hieronymus 2009, p. 21
- ↑ Hieronymus 2009, p. 24
- ↑ Hieronymus 2009, p. 27
- ↑ Hieronymus 2009, p. 28
- ↑ Hieronymus 2009, pp. 221–223
- ↑ Hieronymus 2009, p. 35
- ↑ Hieronymus 2009, p. 36
- ↑ Zhegallo 2005, p. 20
- ↑ MacFadden 2000, p. 226
- ↑ Macfadden 2000, p. 224
- ↑ Koenigswald 1999, pp. 275–276
- ↑ Koenigswald 1999, pp. 281
- ↑ Zhegallo 2005, pp. 34–35
- ↑ Koenigswald 1999, p. 263
- ↑ Wood, Horace Elmer, 2nd (1938). "Causal Factors Shortening Tooth Series with Age" (PDF). Journal of Dental Research. 17 (1): 6–7. doi:10.1177/00220345380170010101.
- ↑ Mendoza, M.; Palmqvist, P. (February 2008). "Hypsodonty in ungulates: an adaptation for grass consumption or for foraging in open habitat?". Journal of Zoology. 273 (2): 134–142. doi:10.1111/j.1469-7998.2007.00365.x.
- ↑ van der Made & Grube 2010, p. 387
- ↑ van der Made & Grube 2010, p. 388
- ↑ Deng & Zheng 2005, p. 120
- ↑ Deng & Zheng 2005, p. 118
- ↑ White rhinoceros (Ceratotherium simum). arkive.org
- ↑ "Forest elephant videos, photos and facts – Loxodonta cyclotis". ARKive. Retrieved 23 July 2012.
- ↑ Macdonald, D. (2001). The New Encyclopedia of Mammals. Oxford: Oxford University Press.
- ↑ Paul, Gregory S. (December 1998). "Limb Designs, Function and Running Performance in Ostrich-mimics and Tyrannosaurs" (PDF). Gaia (15): 258–259. Archived from the original (PDF) on 19 July 2011.
References
- Antoine, Pierre-Olivier (March 2003). "Middle Miocene elasmotheriine Rhinocerotidae from China and Mongolia: taxonomic revision and phylogenetic relationships" (PDF). Zoologica Scripta. The Norwegian Academy of Science and Letters. 32 (2): 95–118. doi:10.1046/j.1463-6409.2003.00106.x.
- Cerdeño, Esperenza (1998). "Diversity and evolutionary trends of the Family Rhinocerotidae (Perissodactyla)" (PDF). Palaeo (141): 13–34.
- Deng, Tao; Zheng, Min (2005). "Limb Bones of Elasmotherium (Rhinocerotidae, Perissodactyla) from Nihewan (Hebei, China)" (PDF). Vertebrata PalAsiatica (in Chinese and English) (4): 110–121.
- Hieronymus, Tobin L. (2009). Osteological Correlates of Cephalic Skin Structures in Amniota: Documenting the Evolution of Display and Feeding Structures with Fossil Data (Doctoral Thesis) (PDF). College of Arts and Sciences of Ohio University.
- v. Koenigswald, Wighart; Goin, Francisco; Pascual, Rosendo (1999). "Hypsodonty and enamel microstructure in the Paleocene gondwanatherian mammal Sudamerica amerghinoi" (PDF). Acta Palaeontologica Polonica. 44 (3): 263–300.
- MacFadden, Bruce J. (2000). "Origin and evolution of the grazing guild in Cenozoic New World terrestrial mammals". In Sues, Hans-Dieter. Evolution of Herbivory in Terrestrial Vertebrates: Perspectives from the Fossil Record. Cambridge: Cambridge University Press. pp. 223–244.
- Russell, James R. (2009). "From Zoroastrian Cosmology and Armenian heresiology to the Russian novel". In Allison, Christine; Joristen-Pruschke, Anke; Wendtland, Antje. From Daēnā to Dîn: Religion, Kultur und Sprache in der iranischen Welt; Festschrift für Philip Kezenbroek zum 60. Geburstag. Wiesbaden: Harrassowitz. pp. 141–208.
- Sinon, Denis (1960). "Sur les noms altaïque de la licorne" (PDF). Wiener Zeitschrift für der Kunde des Morgenlandes (in French) (56): 168–176.
- Titov, V.V. (2008). Late Pliocene large mammals from Northeastern Sea of Azov Region (PDF) (in Russian and English). Rostov-on-Don: SSC RAS Publishing.
- van der Made, Jan; Grube, René (2010). "The rhinoceroses from Neumark-Nord and their nutrition". In Meller, Harald. Elefantenreich – Eine Fossilwelt in Europa (PDF) (in German and English). Halle/Saale. pp. 382–394.
- Zhegallo, V.; Kalandadze, N.; Shapovalov, A.; Bessudnova, Z.; Noskova, N.; Tesakova, E. (2005). "On the fossil rhinoceros Elasmotherium (including the collections of the Russian Academy of Sciences)". Cranium. 22 (1): 17–40.
External links
| Wikimedia Commons has media related to Elasmotherium. |
- Piper, Ross (2010). "Elasmotherium and the case of the massive horn". Scrubmuncher's Blog. Nature Blog Network. Retrieved 30 August 2013.
- Strauss, Bob. "Elasmotherium – About.com Prehistoric Mammals". Dinosaurs at About.com. About.com. Retrieved 30 August 2013.