Chemical field-effect transistor
See also ISFET
A ChemFET is a chemically-sensitive field-effect transistor, that is a field-effect transistor used as a sensor for measuring chemical concentrations in solution.[1] When the target analyte concentration changes, the current through the transistor will change accordingly.[2] Here, the analyte solution separates the source and gate electrodes.[3] A concentration gradient between the solution and the gate electrode arises due to a semi-permeable membrane on the FET surface containing receptor moieties that preferentially bind the target analyte.[3] This concentration gradient of charged analyte ions creates a chemical potential between the source and gate, which is in turn measured by the FET.[4]
Construction
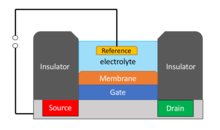
A ChemFET’s source and drain are constructed as for an ISFET, with the gate electrode separated from the source electrode by a solution.[4] The gate electrode’s interface with the solution is a semi-permeable membrane containing the receptors, and a gap to allow the substance under test to come in contact with the sensitive receptor moieties.[5] A ChemFET’s threshold voltage depends on the concentration gradient between the analyte in solution and the analyte in contact with its receptor-embedded semi-permeable barrier.[5]
Often, ionophores are used to facilitate analyte ion mobility through the substrate to the receptor.[6] For example when targeting anions, quaternary ammonium salts (such as tetraoctylammonium bromide) are used to provide cationic nature to the membrane, facilitating anion mobility through the substrate to the receptor moieties.[7]
Applications
ChemFETs can be utilized in either liquid or gas phase to detect target analyte, requiring reversible binding of analyte with a receptor located in the gate electrode membrane.[8][3] There is a wide range of applications of ChemFETs, including most notably anion or cation selective sensing.[5] More work has been done with cation-sensing ChemFETs than anion-sensing ChemFETs.[5] Anion-sensing is more complicated than cation-sensing in ChemFETs due to many factors, including the size, shape, geometry, polarity, and pH of the species of interest.[5]
Practical Limitations
The body of a ChemFET is generally found to be robust.[9][4] However, the unavoidable requirement for a separate reference electrode makes the system more bulky overall and potentially more fragile.[10]
History
ChemFETs are based on a modified ISFET, a concept developed by Bergveld in the 1970s.[4] The dividing line between ChemFET and ISFET is unclear. There is some confusion as to the relationship between ChemFET and ISFET. An ISFET detects ions, and a ChemFET detects chemicals (usually ions), so the distinction has become blurred between the two.
See also
References
- ↑ Reinhoudt, David N. "Application of supramolecular chemistry in the development of ion-selective CHEMFETs". Sensors and Actuators B: Chemical. 6 (1–3): 179. doi:10.1016/0925-4005(92)80052-y.
- ↑ Lugtenberg, Ronny J. W.; Antonisse, Martijn M. G.; Egberink, Richard J. M.; Engbersen, Johan F. J.; Reinhoudt, David N. (1996-01-01). "Polysiloxane based CHEMFETs for the detection of heavy metal ions". Journal of the Chemical Society, Perkin Transactions 2. 0 (9). doi:10.1039/p29960001937. ISSN 1364-5471.
- 1 2 3 Janata, Jiri (2004-11-01). "Thirty Years of CHEMFETs – A Personal View". Electroanalysis. 16 (22): 1831. doi:10.1002/elan.200403070. ISSN 1521-4109.
- 1 2 3 4 Bergveld, P. "Thirty years of ISFETOLOGY". Sensors and Actuators B: Chemical. 88 (1): 1. doi:10.1016/s0925-4005(02)00301-5.
- 1 2 3 4 5 Antonisse, Martijn M. G.; Reinhoudt, David N. (1999-10-01). "Potentiometric Anion Selective Sensors". Electroanalysis. 11 (14): 1035. doi:10.1002/(sici)1521-4109(199910)11:14%3C1035::aid-elan1035%3E3.0.co;2-i. ISSN 1521-4109.
- ↑ Wróblewski, Wojciech; Wojciechowski, Kamil; Dybko, Artur; Brzózka, Zbigniew; Egberink, Richard J.M; Snellink-Ruël, Bianca H.M; Reinhoudt, David N. "Durability of phosphate-selective CHEMFETs". Sensors and Actuators B: Chemical. 78 (1–3): 315–319. doi:10.1016/s0925-4005(01)00832-2.
- ↑ Antonisse, Martijn M. G.; Snellink-Ruël, Bianca H. M.; Engbersen, Johan F. J.; Reinhoudt, David N. (1998-01-01). "Chemically modified field effect transistors with nitrite or fluoride selectivity". Journal of the Chemical Society, Perkin Transactions 2. 0 (4): 775. doi:10.1039/a709076e. ISSN 1364-5471.
- ↑ Han, Jin-Woo; Rim, Taiuk; Baek, Chang-Ki; Meyyappan, M. (2015-09-30). "Chemical Gated Field Effect Transistor by Hybrid Integration of One-Dimensional Silicon Nanowire and Two-Dimensional Tin Oxide Thin Film for Low Power Gas Sensor". ACS Applied Materials & Interfaces. 7 (38): 21263. doi:10.1021/acsami.5b05479. ISSN 1944-8244.
- ↑ Jimenez-Jorquera, Cecilia; Orozco, Jahir; Baldi, Antoni (2009-12-24). "ISFET Based Microsensors for Environmental Monitoring". Sensors. 10 (1): 66. doi:10.3390/s100100061.
- ↑ "ISFET". Wikipedia. 2017-11-10.