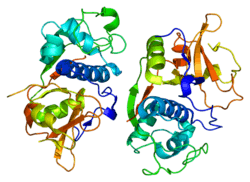Cathepsin F
Cathepsin F is a protein that in humans is encoded by the CTSF gene.[5][6][7]
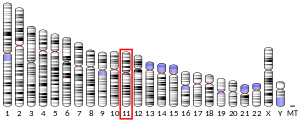
Cathepsins are papain family cysteine proteinases that represent a major component of the lysosomal proteolytic system. In general, cathepsins contain a signal sequence, followed by a propeptide and then a catalytically active mature region. The very long (251-amino acid residues) proregion of the cathepsin F precursor contains a C-terminal domain similar to the pro-segment of cathepsin L-like enzymes, a 50-residue flexible linker peptide, and an N-terminal domain predicted to adopt a cystatin-like fold. The cathepsin F proregion is unique within the papain family cysteine proteases in that it contains this additional N-terminal segment predicted to share structural similarities with cysteine protease inhibitors of the cystatin superfamily. This cystatin-like domain contains some of the elements known to be important for inhibitory activity. CTSF encodes a predicted protein of 484 amino acids that contains a 19-residue signal peptide. Cathepsin F contains five potential N-glycosylation sites, and it may be targeted to the endosomal/lysosomal compartment via the mannose 6-phosphate receptor pathway. The cathepsin F gene is ubiquitously expressed, and it maps to chromosome 11q13, close to the gene encoding cathepsin W.[7]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000174080 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000083282 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Wang B, Shi GP, Yao PM, Li Z, Chapman HA, Bromme D (Dec 1998). "Human cathepsin F. Molecular cloning, functional expression, tissue localization, and enzymatic characterization". J Biol Chem. 273 (48): 32000–8. doi:10.1074/jbc.273.48.32000. PMID 9822672.
- ↑ Santamaria I, Velasco G, Pendas AM, Paz A, Lopez-Otin C (Jun 1999). "Molecular cloning and structural and functional characterization of human cathepsin F, a new cysteine proteinase of the papain family with a long propeptide domain". J Biol Chem. 274 (20): 13800–9. doi:10.1074/jbc.274.20.13800. PMID 10318784.
- 1 2 "Entrez Gene: CTSF cathepsin F".
Further reading
- Nägler DK, Sulea T, Ménard R (1999). "Full-length cDNA of human cathepsin F predicts the presence of a cystatin domain at the N-terminus of the cysteine protease zymogen". Biochem. Biophys. Res. Commun. 257 (2): 313–8. doi:10.1006/bbrc.1999.0461. PMID 10198209.
- Wex T, Levy B, Wex H, Brömme D (1999). "Human cathepsins F and W: A new subgroup of cathepsins". Biochem. Biophys. Res. Commun. 259 (2): 401–7. doi:10.1006/bbrc.1999.0700. PMID 10362521.
- Wex T, Wex H, Brömme D (2000). "The human cathepsin F gene--a fusion product between an ancestral cathepsin and cystatin gene". Biol. Chem. 380 (12): 1439–42. doi:10.1515/BC.1999.185. PMID 10661872.
- Shi GP, Bryant RA, Riese R, et al. (2000). "Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages". J. Exp. Med. 191 (7): 1177–86. doi:10.1084/jem.191.7.1177. PMC 2193169. PMID 10748235.
- Deussing J, Tisljar K, Papazoglou A, Peters C (2000). "Mouse cathepsin F: cDNA cloning, genomic organization and chromosomal assignment of the gene". Gene. 251 (2): 165–73. doi:10.1016/S0378-1119(00)00196-7. PMID 10876093.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Oörni K, Sneck M, Brömme D, et al. (2004). "Cysteine protease cathepsin F is expressed in human atherosclerotic lesions, is secreted by cultured macrophages, and modifies low density lipoprotein particles in vitro". J. Biol. Chem. 279 (33): 34776–84. doi:10.1074/jbc.M310814200. PMID 15184381.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Vazquez-Ortiz G, Pina-Sanchez P, Vazquez K, et al. (2006). "Overexpression of cathepsin F, matrix metalloproteinases 11 and 12 in cervical cancer". BMC Cancer. 5 (1): 68. doi:10.1186/1471-2407-5-68. PMC 1175083. PMID 15989693.
- Kaakinen R, Lindstedt KA, Sneck M, et al. (2007). "Angiotensin II increases expression and secretion of cathepsin F in cultured human monocyte-derived macrophages: an angiotensin II type 2 receptor-mediated effect". Atherosclerosis. 192 (2): 323–7. doi:10.1016/j.atherosclerosis.2006.08.001. PMID 16963053.
External links