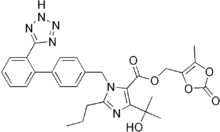
Olmesartan
 | |
| Clinical data | |
|---|---|
| Trade names | Benicar |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603006 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 26% |
| Metabolism | Hepatic (cannot be removed by hemodialysis) |
| Elimination half-life | 13 hours |
| Excretion | Renal 40%, biliary 60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.174.243 |
| Chemical and physical data | |
| Formula | C29H30N6O6 |
| Molar mass | 558.585 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Olmesartan medoxomil is an angiotensin II receptor antagonist which is used for the treatment of high blood pressure. It was developed by Sankyo in 1995, and is sold under the trade name Benicar, among others. An ester prodrug, it is completely and rapidly hydrolyzed to the active acid form, olmesartan (RNH-6270).[1]
Indications
Olmesartan is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.[2] The U.S. Food and Drug Administration (FDA) has determined that the benefits of Benicar continue to outweigh its potential risks when used for the treatment of patients with high blood pressure according to the drug label.[3]
Contraindications
Contraindications for treatment with olmesartan include biliary obstruction. Another major contraindication is pregnancy; reports in the scientific literature reveal fetal malformations for pregnant women taking sartan-derived drugs.[4]
Adverse effects
The incidence of adverse effects with Benicar (the US trade name for olmesartan medoxomil) is reported as similar to placebo; the only adverse effect that occurred in >1% of patients treated with it and more frequently than placebo was dizziness (3% vs 1%). The full prescribing information for Benicar notes as with all drugs that act directly on the renin-angiotensin system, olmesartan is contraindicated in pregnancy and can cause injury and even death to the developing fetus. In studies of angiotensin II receptor antagonists such as olmesartan, patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen have been reported. There has been no long-term use of olmesartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected.[5] Rarely, olmesartan can cause severe gastrointestinal issues. The symptoms, which include nausea, vomiting, diarrhea, weight loss, and electrolyte abnormalities, are common among those who have celiac disease.[6] Recent studies suggested this form of sprue-like enteropathy could be caused by the inhibition of TGF-β, a polypeptide cytokine that maintains intestinal homeostasis. However, it is still unclear why this action was never observed with other ARBs.[7]
Dosage and administration
The usual recommended starting dose of olmesartan is 20 mg once daily. The dose may be increased to 40 mg after two weeks of therapy, if further reduction in blood pressure is desirable. Doses above 40 mg do not appear to have greater effect, and twice-daily dosing offers no advantage over the same total dose given once daily.[2] No adjustment of dosage is typically necessary for advanced age, renal impairment, or hepatic dysfunction. For patients with possible depletion of intravascular volume (e.g., patients treated with diuretics), olmesartan should be initiated with caution; consideration should be given to use of a lower starting dose in such cases.[2] If blood pressure is not controlled by Benicar alone, a diuretic may be added. Benicar may be administered with other antihypertensive agents. Benicar may be administered with or without food.[2]
Preparations
Olmesartan and Sevikar HCT combined is marketed worldwide by Daiichi Sankyo, in India by Abbott Healthcare Pvt. Ltd. under the trade name WinBP, by Zydus Cadila under the trade name Olmy, by Ranbaxy Laboratories Ltd. under the trade name Olvance, Olsar by Unichem Laboratories and in Canada by Schering-Plough as Olmetec. Several preparations containing olmesartan and other antihypertensives are available. Teva Pharmaceuticals produces a formulation containing olmesartan, amlodipine, and hydrochlorothiazide for once daily use.[8] Benicar HCT is the brand name of a medication containing olmesartan medoxomil in combination with hydrochlorothiazide. Benitec H, another medication containing olmesartan medoxomil and hydrochlorothiazide, is marketed by GlaxoSmithKline in India.
Research
Two clinical studies (MORE [9] and OLIVUS [10])[11] report that Benicar reduced arterial plaque during therapy for high blood pressure.
In a small study with 44 patients with chronic kidney disease without a history of diabetes, olmesartan was more effective in reducing daily urinary protein (proteinuria) than losartan, valsartan, and candesartan.[12]
See also
References
- ↑ Aulakh GK, Sodhi RK, Singh M (August 2007), "An update on non-peptide angiotensin receptor antagonists and related RAAS modulators", Life Sci., 81 (8): 615–39, doi:10.1016/j.lfs.2007.06.007, PMID 17692338
- 1 2 3 4 RxList Inc. (5 July 2007). "Benicar (olmesartan medoxomil)". RxList Inc. Retrieved 22 July 2010. External link in
|publisher=(help) - ↑ "FDA Alert: Benicar (olmesartan): Ongoing Safety Review". Drugs.com. Retrieved 2013-06-27.
- ↑ Hünseler, C; Paneitz, A; Friedrich, D; Lindner, U; Oberthuer, A; Körber, F; Schmitt, K; Welzing, L; Müller, A; Herkenrath, P; Hoppe, B; Gortner, L; Roth, B; Kattner, E; Schaible, T (Jan 2011). "Angiotensin II receptor blocker induced fetopathy: 7 cases". Klin Padiatr. 223 (1): 10–4. doi:10.1055/s-0030-1269895.
- ↑ "BENICAR Prescribing Information" (PDF). Archived from the original (PDF) on 2010-12-13. Retrieved 2011-01-20.
- ↑ De Petris G, Caldero SG, Chen L, et al. (May 2014). "Histopathological changes in the gastrointestinal tract due to medications: an update for the surgical pathologist (part II of II)". Int. J. Surg. Pathol. 22 (3): 202–11. doi:10.1177/1066896913502230. PMID 24021900.
- ↑ Rubio-Tapia, Alberto; Herman, Margot L.; Ludvigsson, Jonas F.; Kelly, Darlene G.; Mangan, Thomas F.; Wu, Tsung-Teh; Murray, Joseph A. (2012-08-01). "Severe Spruelike Enteropathy Associated With Olmesartan". Mayo Clinic Proceedings. 87 (8): 732–738. doi:10.1016/j.mayocp.2012.06.003. ISSN 0025-6196. PMC 3538487. PMID 22728033.
- ↑ https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c128ef2-60a6-40f3-b37f-ee139fe27987
- ↑ as referenced in http://www.medicalnewstoday.com/releases/91285.php "Olmetec(R) Is First Angiotensin Receptor Blocker (ARB) To Suggest Atherosclerosis Regression (In Hypertensives With Cardiovascular Risk), UK"
- ↑ Cardiovascular Research Foundation (2008, October 16). Drug May Reduce Coronary Artery Plaque. ScienceDaily. Retrieved January 5, 2013, from https://www.sciencedaily.com/releases/2008/10/081012121318.htm
- ↑ (Review) R Preston Mason, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, and Elucida Research, Beverly, MA, USA. Vascular Health and Risk Management, Dovepress, Published Date June 2011 Volume 2011:7 Pages 405 - 416. Optimal therapeutic strategy for treating patients with hypertension and atherosclerosis: focus on olmesartan medoxomil. Retrieved January 5, 2013, from http://www.dovepress.com/optimal-therapeutic-strategy-for-treating-patients-with-hypertension-a-peer-reviewed-article-VHRM
- ↑ "Olmesartan is More Effective Than Other Angiotensin Receptor Antagonists in Reducing Proteinuria in Patients With Chronic Kidney Disease Other Than Diabetic Nephropathy". Retrieved September 20, 2016.
External links
- Daiichi-Sankyo Benicar page
- Benicar HCT from RXlist.com
- Mayo Clinic Proceedings vol.87 Issue 8, pages 732-738