Arsonic acids
Arsonic acids are a subset of organoarsenic compounds defined as oxyacids where a pentavalent arsenic atom is bonded to two hydroxyl groups, a third oxygen atom (this one with a double bond), and an organic substituent. The salts/conjugate bases of arsonic acids are called arsonates. Like all arsenic-containing compounds, arsonic acids are toxic and carcinogenic to humans.[1][2]
Arsonic acid refers to H3AsO3, the case where the substituent is a single hydrogen atom. The other arsonic acids can simply be viewed as hydrocarbyl derivatives of this base case. Arsenic acid results when the substituent is a hydroxyl group. Methylarsonic acid results when the substituent is a methyl group. Phenylarsonic acid results when the substituent is a phenyl group.
Uses
Poultry feed
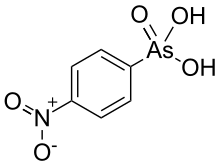
Arsanilic acid, carbarsone, nitarsone, and roxarsone were formerly used in poultry feed in order to promote growth and increase feed conversion.[3][4] In addition, nitarsone and carbarsone can also prevent histomoniasis.[5][6][7] However, concern grew over whether or not the arsenic would be ingested by humans when they ate the poultry. In 2013, a study found that chickens who were fed roxarsone and other arsenic-containing feed additives tended to show elevated levels of arsenic in breast meat—three times as high—compared to chickens that were not fed any arsenical feed additives.[8][9] On September of that year, Zoetis and Fleming Laboratories, the drugs' sponsors, voluntarily withdrew the FDA approvals for arsanilic acid, carbarsone, nitarsone, and roxarsone,[10] leaving only nitarsone approved until its approval for use in animal feed was withdrawn by the FDA in 2015.[11]
Medicine
Difetarsone and carbarsone can be used to treat protozoal infections and Entamoeba histolytica infections.[12][13][14][15] Difetarsone can also be used to treat whipworm infections.[16] Arsanilic acid was discovered to treat sleeping sickness in the early 1900s,[17] but its usage in humans was discontinued after it was found to be too toxic.[18] Acetarsol is an anti-infective.[19]
List
Arsenic acid is technically not an arsonic acid because the substituent is a hydroxyl group, not a hydrocarbyl group, so arsenic acid has three hydroxyl groups bound to the arsenic atom, while arsenic atoms only have two.
| Name | Picture | Substituent |
|---|---|---|
| Arsonic acid | Hydrogen atom | |
| Arsenic acid | 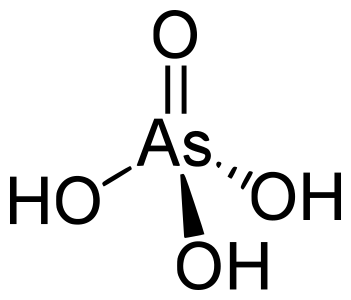 | Hydroxyl group |
| Acetarsol | 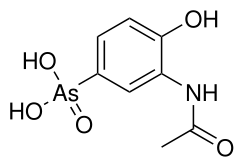 | ''ortho''-acetamol |
| Arsanilic acid | 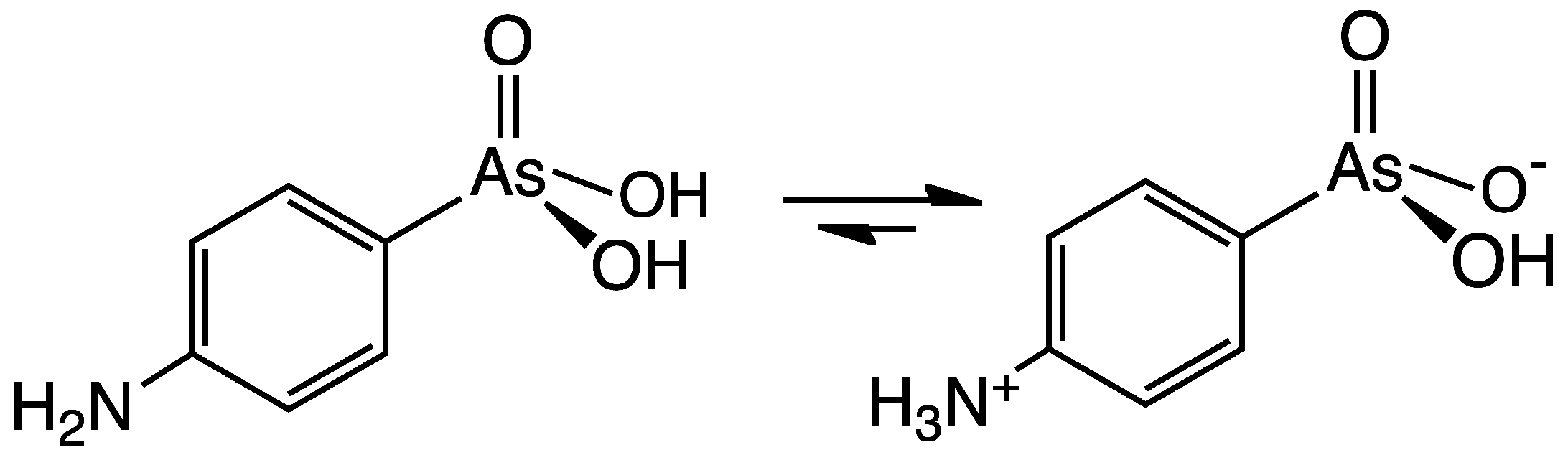 | Aniline |
| Methylarsonic acid | 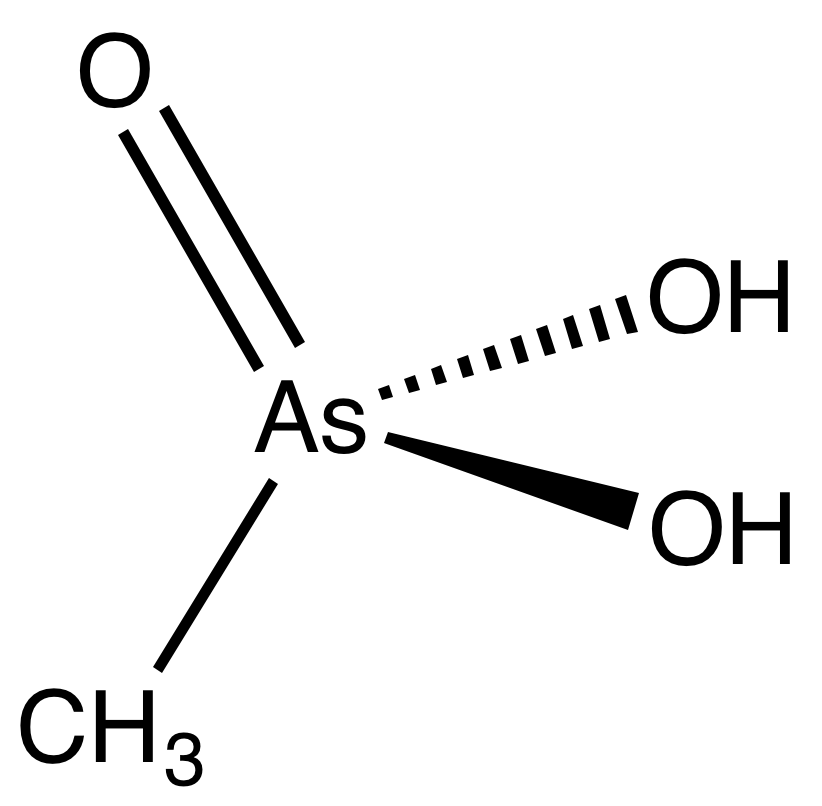 | Methyl group |
| Phenylarsonic acid | 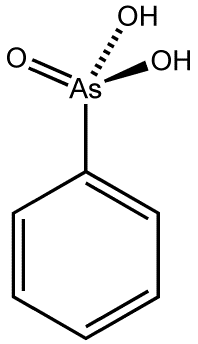 | Phenyl group |
| P-Azobenzenearsonate | [[Image:]] | |
| Carbarsone | 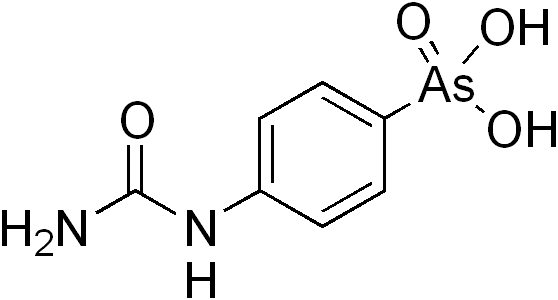 | |
| Difetarsone | [[Image:]] | |
| Nitarsone | [[Image:]] | |
| Thorin (chemistry) | [[Image:]] | |
| Roxarsone | [[Image:]] |
References
- ↑ Aronson, Jeffrey K. (2009). Meyler's Side Effects of Antimicrobial Drugs. p. 834. ISBN 9780080932934. Retrieved October 7, 2013.
- ↑ Styblo, M.; Del Razo, L. M.; Vega, L.; Germolec, D. R.; LeCluyse, E. L.; Hamilton, G. A.; Reed, W.; Wang, C.; Cullen, W. R.; Thomas, D. J. (2000). "Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells". Archives of Toxicology. 74: 289–299. doi:10.1007/s002040000134.
- ↑ Levander OA, ed. (1977). "Biological effects of arsenic on plants and animals: Domestic animals: Phenylarsonic feed additives". Arsenic: Medical and Biological Effects of Environmental Pollutants. Washington DC: National Academies Press. pp. 149–51. ISBN 978-0-309-02604-8.
- ↑ "Arsanilic acid—MIB #4". Canadian Food Inspection Agency. Sep 2006. Archived from the original on 2012-12-13. Retrieved 3 Aug 2012.
- ↑ U.S. Food and Drug Administration. "Animal Drugs @ FDA".
- ↑ McDougald LR (1979). "Efficacy and compatibility of amprolium and carbarsone against Coccidiosis and blackhead in turkeys". Poult. Sci. 58 (1): 76–80. doi:10.3382/ps.0580076. PMID 572970.
- ↑ Worden AN, Wood EC (1973). "The effect of Carbarsone (33.6 per cent w-v p-ureidobenzene arsonic acid) on bodyweight gain, food conversion and tissue arsenic levels of turkey poults". J. Sci. Food Agric. 24 (1): 35–41. doi:10.1002/jsfa.2740240107. PMID 4696593.
- ↑ KE Nachman; PA Baron; G Raber; KA Francesconi; A Navas-Acien; DC Love (2013). "Roxarsone, Inorganic Arsenic, and Other Arsenic Species in Chicken: A U.S.-Based Market Basket Sample" (PDF). Environmental Health Perspectives. 121: 818–824. doi:10.1289/ehp.1206245.
- ↑ Sabrina Tavernise (May 11, 2013). "Study Finds an Increase in Arsenic Levels in Chicken". New York Times.
- ↑ U.S. Food and Drug Administration (1 Oct 2013). "FDA response to citizen petition on arsenic-based animal drugs".
- ↑ U.S. Food and Drug Administration (April 1, 2015). "FDA announces pending withdrawal of approval of nitarsone".
- ↑ SASAKI T, YOKAGAWA M, WYKOFF DE, RITICHIE LS (1956). "Asymptomatic amebiasis; treatment with atabrine in combination with carbarsone or chiniofon". United States Armed Forces medical journal. 7 (3): 363–8. PMID 13299463.
- ↑ RADKE RA (1955). "Ameboma of the intestine: an analysis of the disease as presented in 78 collected and 41 previously unreported cases". Ann. Intern. Med. 43 (5): 1048–66. doi:10.7326/0003-4819-43-5-1048. PMID 13268997.
- ↑ HOEKENGA MT (1 July 1951). "A comparison of aureomycin and carbarsone in the treatment of intestinal amebiasis". Am. J. Trop. Med. Hyg. 31 (4): 423–5. PMID 14857246.
- ↑ Aronson, Jeffrey K. (2009). Meyler's Side Effects of Antimicrobial Drugs. p. 834. ISBN 9780080932934. Retrieved October 7, 2013.
- ↑ P.M. Leary; C. Jones; F. Douglas; S.T. Boyd (June 1972). "Difetarsone in Difetarsone in outpatient treatment of Trichuris trichiura infestation". Archives of Disease in Childhood. 49 (6): 486–8. doi:10.1136/adc.49.6.486. PMC 1648795. PMID 4851370.
- ↑ Boyce R (1907). "The treatment of sleeping sickness and other trypanosomiases by the Atoxyl and mercury method". BMJ. 2 (2437): 624–5. doi:10.1136/bmj.2.2437.624. PMC 2358391. PMID 20763444.
- ↑ Burke ET (1925). "The arseno-therapy of syphilis; stovarsol, and tryparsamide". British Journal of Venereal Diseases. 1 (4): 321–38. doi:10.1136/sti.1.4.321. PMC 1046841. PMID 21772505.
- ↑ Chen MY, Smith NA, Fox EF, Bingham JS, Barlow D (April 1999). "Acetarsol pessaries in the treatment of metronidazole resistant Trichomonas vaginalis". Int J STD AIDS. 10 (4): 277–80. doi:10.1258/0956462991913943. PMID 12035784.