Antibody-dependent cell-mediated cytotoxicity
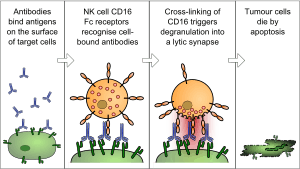
The antibody-dependent cell-mediated cytotoxicity (ADCC), also referred to as antibody-dependent cellular cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies.[1] It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.[2]
ADCC is independent of the immune complement system that also lyses targets but does not require any other cell. ADCC requires an effector cell which classically is known to be natural killer (NK) cells that typically interact with IgG antibodies.[3] However, macrophages, neutrophils and eosinophils can also mediate ADCC, such as eosinophils killing certain parasitic worms known as helminths via IgE antibodies.[4]
ADCC is part of the adaptive immune response due to its dependence on a prior antibody response. The coating of target cells with antibodies is sometimes referred to as opsonization.
By NK cells
The typical ADCC involves activation of NK cells by antibodies. A NK cell expresses Fc receptors, mostly CD16. These receptors recognize, and bind to, the Fc portion of an antibody, such as IgG, which has bound to the surface of a pathogen-infected target cell. The most common Fc receptor on the surface of an NK cell is called CD16 or FcγRIII. Once the Fc receptor binds to the Fc region of IgG, the Natural Killer cell releases cytotoxic factors that cause the death of the target cell.
During replication of a virus some of the viral proteins are expressed on the cell surface membrane of the infected cell. Antibodies can then bind to these viral proteins. Next, the NK cells which have Fc Receptors will bind to that antibody, inducing the NK cell to release proteins such as perforin and proteases known as granzymes, which causes the lysis of the infected cell to hinder the spread of the virus.
Furthermore, NK cells are involved in killing tumor cells and other cells that may lack MHC I on their surface, indicating a non-self cell. This is because, generally, all nucleated cells (which excludes RBCs) of the body contain MHC I.
By eosinophils
Large parasites like helminths are too big to be engulfed and killed by phagocytosis. They also have an external structure or integument that is resistant to attack by substances released by neutrophils and macrophages. After IgE coat these parasites, the Fc receptor (FceRI) of an eosinophil will then recognize IgE. Subsequently, interaction between FceRI and the Fc portion of helminth-bound IgE signals the eosinophil to degranulate.
In vitro Assays
Several laboratory methods exist for determining the efficacy of antibodies or effector cells in eliciting ADCC. Usually, a target cell line expressing a certain surface-exposed antigen is incubated with antibody specific for that antigen. After washing, effector cells expressing Fc receptor CD16 are co-incubated with the antibody-labelled target cells. Effector cells are typically PBMCs (peripheral blood mononuclear cell), of which a small percentage are NK cells (Natural Killer cell); less often they are purfied NK cells themselves. Over the course of a few hours a complex forms between the antibody, target cell, and effector cell which leads to lysis of the cell membrane of the target. If the target cell was pre-loaded with a label of some sort, that label is released in proportion to the amount of cell lysis. Cytotoxicity can be quantified by measuring the amount of label in solution compared to the amount of label that remains within healthy, intact cells.
The classical method of detecting this is the Chromium-51 [51Cr] release assay; the Sulfur-35 [35S] release assay is a little used radioisotope-based alternative. Target cell lysis is determined by measuring the amount of radiolabel released into the cell culture medium by means of a gamma counter or scintillation counter. A variety of non-radioactive methods are now in widespread use. Fluorescence-based methods include such things as direct labeling with a fluorescent dye like calcein or labeling with europium that becomes fluorescent when released Eu3+ binds to a chelator. Fluorescence can be measured by means of multi-well fluorometers or by flow cytometry methods. There are also enzymatic-based assays in which the contents of the lysed cells includes cellular enzymes like GAPDH that remain active; supplying a substrate for that enzyme can catalyze a reaction whose product can be detected by luminescence or by absorbance.
Monoclonal antibody action against cancer
The effects against solid tumors of trastuzumab and rituximab monoclonal antibodies have been shown in experiments with mice to involve ADCC as an important mechanism of therapeutic action.[5] In the clinic, the FcgRIII 158V/F polymorphism interfere with the ability to generate ADCC responses in vitro during trastuzumab treatment.
Multiple myeloma can be treated with daratumumab (Darzalex) monoclonal antibody.[6] Studies with in vitro materials and patient materials indicate that ADCC is an important mechanism, along with CDC (Complement-dependent cytotoxicity).[7]
See also
References
- ↑ Hashimoto, G.; Wright, P. F.; Karzon, D. T. (1983-11-01). "Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells". The Journal of Infectious Diseases. 148 (5): 785–794. doi:10.1093/infdis/148.5.785. ISSN 0022-1899. PMID 6605395.
- ↑ Pollara, Justin; Hart, Lydia; Brewer, Faraha; Pickeral, Joy; Packard, Beverly Z.; Hoxie, James A.; Komoriya, Akira; Ochsenbauer, Christina; Kappes, John C. (2011-08-01). "High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses". Cytometry Part A. 79 (8): 603–612. doi:10.1002/cyto.a.21084. ISSN 1552-4930. PMC 3692008. PMID 21735545.
- ↑ Wang, W; Erbe, AK; Hank, JA; Morris, ZS; Sondel, PM (2015). "NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy". Front Immunol. 6: 368. doi:10.3389/fimmu.2015.00368. PMC 4515552. PMID 26284063.
- ↑ Capron, M; Kazatchkine, MD; Fischer, E; Joseph, M; Butterworth, AE; et al. (1987). "Functional role of the alpha-chain of complement receptor type 3 in human eosinophil-dependent antibody-mediated cytotoxicity against schistosomes". J Immunol. 139 (6): 2059–65. PMID 2957447.
- ↑ Clynes, RA; Towers, TL; Presta, LG; Ravetch, JV (2000). "Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets". Nat Med. 6 (4): 443–6. doi:10.1038/74704. PMID 10742152.
- ↑ Sanchez, L; Wang, Y; Siegel, DS (2016). "Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma". J Hematol Oncol. 9 (1): 51. doi:10.1186/s13045-016-0283-0. PMC 4929758. PMID 27363983.
- ↑ de Weers, M; Tai, YT; Bakker, JM; Vink, T; Jacobs, DC; et al. (2011). "Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors". J Immunol. 186 (3): 1840–8. doi:10.4049/jimmunol.1003032. PMID 21187443. Retrieved 28 April 2017.
Further reading
- Janeway CA Jr.; et al. (2001). Immunobiology (5th ed.). Garland Publishing. ISBN 0-8153-3642-X. (electronic full text via NCBI Bookshelf).
- Pier GB, Lyczak JB, Wetzler LM (2004). Immunology, Infection, and Immunity. ASM Press. ISBN 1-55581-246-5.
External links
- University of Leicester, Virus Immunopathology Notes
- Antibody-Dependent+Cell+Cytotoxicity at the US National Library of Medicine Medical Subject Headings (MeSH)