Angiotensin II (medication)
 | |
| Clinical data | |
|---|---|
| Trade names | Giapreza |
| AHFS/Drugs.com | Professional Drug Facts |
| Routes of administration | Intravenous injection |
| Drug class | Vasoconstrictor |
| Pharmacokinetic data | |
| Elimination half-life | less than one minute (IV administration) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| Chemical and physical data | |
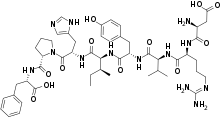
| Formula | C50H71N13O12 |
| Molar mass | 1,046.20 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Angiotensin II is a medication that is used to treat hypotension resulting from septic shock. It is a synthetic vasoconstrictor peptide that is identical to human hormone angiotensin II[1] and is marketed under the brandname Giapreza. The Food and Drug Administration approved the use of Giapreza in December of 2017 to treat low blood pressure resulting from septic shock.[2]
References
- ↑ Kaufman MB (March 2018). "Pharmaceutical Approval Update". P & T : a Peer-reviewed Journal for Formulary Management. 43 (3): 141–170. PMID 29491694.
- ↑ "FDA approves drug to treat dangerously low blood pressure". United States Food and Drug Administration. 21 December 2017.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.