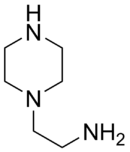
Aminoethylpiperazine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Piperazin-1-ylethanamine | |
| Other names
2-(1-Piperazinyl)ethylamine, AEP, N-AEP, N-(2-Aminoethyl)piperazine, 2-Piperazinoethylamine, 1-(2-Aminoethyl)piperazine, 1-Piperazine ethanamine, 1-Aminoethylpiperazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.920 |
| EC Number | 205-411-0 |
PubChem CID |
|
| |
| |
| Properties | |
| C6H15N3 | |
| Molar mass | 129.21 g·mol−1 |
| Appearance | Colourless to yellowish liquid |
| Density | 0.984 g/cm3 at 20 °C |
| Melting point | −19 °C (−2 °F; 254 K) |
| Boiling point | 222 °C (432 °F; 495 K) |
| Fully miscible | |
| Vapor pressure | 0.076 mmHg @ 20 °C |
| Hazards | |
| Main hazards | harmful, corrosive, sensitizing |
| R-phrases (outdated) | R21 R22 R43 R52 R53 |
| S-phrases (outdated) | S26 S36 S37 S39 S45 S61 |
| NFPA 704 | |
| Flash point | 93 °C (199 °F; 366 K) |
| 315 °C (599 °F; 588 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aminoethylpiperazine is a derivative of piperazine. This ethyleneamine contains three nitrogen atoms; one primary, one secondary and one tertiary. It is a corrosive liquid and can cause second or third degree burns. Aminoethylpiperazine can also cause pulmonary edema as a result of inhalation. Uses include inhibition of corrosion, epoxy curing, surface activation, and as an asphalt additive. When used as an epoxy resin curing agent, it is usually used in conjunction with other amines as an accelerator as it only has 3 amine hydrogens for cross-linking.
See also
External links
- Catalytic method for the conjoint manufacture of N-aminoethylpiperazine
- Safety MSDS Data
- Safety data sheet
- Data sheet
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.
