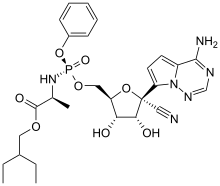
Remdesivir
Remdesivir is an antiviral medication developed by the American biopharmaceutical company Gilead Sciences. It is a nucleotide analog, specifically an adenosine analogue, which inserts into viral RNA chains, causing their premature termination. It was studied during 2020 as a possible post-infection treatment for COVID-19.[1]
 | |
| Clinical data | |
|---|---|
| Other names | GS-5734 |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C27H35N6O8P |
| Molar mass | 602.585 g·mol−1 |
| 3D model (JSmol) | |
| |
InChI
| |
Research
Remdesivir was created and developed by Gilead Sciences, under the direction of Tomáš Cihlář,[2] as a treatment for Ebola virus disease and Marburg virus infections.[3] Gilead Sciences subsequently discovered that remdesivir had antiviral activity in vitro against multiple filo-, pneumo-, paramyxo-, and corona- viruses.[4]
COVID-19
As of April 2020, remdesivir was viewed as the most promising treatment for COVID-19 by Johns Hopkins University.[5] Data from one randomized controlled trial was released early in error and before peer review; it did not show improvement. Gilead Sciences stated that due to low enrollment the study was halted while a non-associated researcher stated it does mean if there is any benefit, then that benefit will be small.[6] Other clinical trials were underway or planned.[7][8][9][10][11][12][13][14][15][16][17]
On 18 March 2020, the World Health Organization (WHO) announced the launch of a trial that would include one group treated with remdesivir.[18][19] While a cohort study published in April 2020, saw possible improvement, determining whether or not the medication is effective will require a randomized controlled trial.[20]
In January 2020, Gilead began laboratory testing of remdesivir against SARS-CoV-2, stating that remdesivir had been shown to be active against severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) in animal models.[21][22][23] In March 2020, a small trial of remdesivir in rhesus macaque monkeys with COVID-19 infections found that it prevents disease progression.[24][25] On 21 January 2020, the Wuhan Institute of Virology applied for a Chinese "use patent", for treating COVID-19.[26]
Ebola
On 9 October 2015, the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) announced preclinical results that remdesivir had blocked the Ebola virus in Rhesus monkeys. Travis Warren, who has been a USAMRIID principal investigator since 2007, said that the "work is a result of the continuing collaboration between USAMRIID and Gilead Sciences".[27] The "initial screening" of the "Gilead Sciences compound library to find molecules with promising antiviral activity" was performed by scientists at the Centers for Disease Control and Prevention (CDC).[27] As a result of this work, it was recommended that remdesivir "should be further developed as a potential treatment."[27][3]
Remdesivir was rapidly pushed through clinical trials due to the West African Ebola virus epidemic of 2013–2016, eventually being used in people with the disease. Preliminary results were promising; it was used in the emergency setting during the Kivu Ebola epidemic that started in 2018, along with further clinical trials, until August 2019, when Congolese health officials announced that it was significantly less effective than monoclonal antibody treatments such as mAb114 and REGN-EB3. The trials, however, established its safety profile.[28][29][30][3][31][32][33][34]
Access
On 17 March 2020, the drug was provisionally approved for use for COVID-19 patients in a serious condition as a result of the outbreak in the Czech Republic.[35] On 20 March 2020, United States President Donald Trump announced that remdesivir was available for "compassionate use" by people with COVID-19; FDA Commissioner Stephen Hahn confirmed the statement at the same press conference.[36] On 23 March 2020, Gilead voluntarily suspended access for compassionate use (excepting cases of critically ill children and pregnant women), for reasons related to supply, citing the need to continue to provide the agent for testing in clinical trials.[37][38]
As of 11 April 2020, access in Canada was only to those who will be involved in a clinical trial.[39]
Mechanism of action and resistance
Remdesivir is a prodrug that metabolizes into its active form GS-441524. An adenosine nucleotide analog, GS-441524 interferes with the action of viral RNA-dependent RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production.[40] Though in some viruses, such as the respiratory syncytial virus but not Ebola virus, it causes the RNA-dependent RNA polymerases to pause, its predominant effect is to induce an irreversible chain termination. Unlike with many other chain terminators, this was not mediated by preventing addition of the immediately subsequent nucleotide, but is instead delayed, occurring after five additional bases have been added to growing RNA chain.[41]
Mutations in the mouse hepatitis virus RNA replicase that cause partial resistance to remdesivir were identified in 2018. These mutations make the viruses less effective in nature, and the researchers believe they will likely not persist where the drug is not being used.[40]
Synthesis
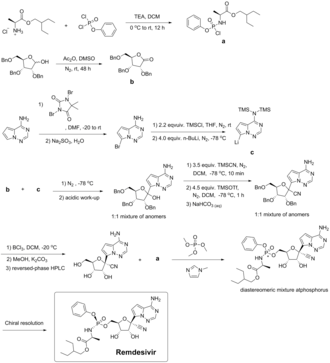
Remdesivir can be synthesized in multiple steps from ribose derivatives. The figure to the right is one of the synthesis routes of remdesivir invented by Chun and coauthors from Gilead Sciences.[42] In this method, intermediate a is firstly prepared from L-alanine and phenyl phosphorodichloridate in presence of triethylamine and dichloromethane; triple benzyl-protected ribose is oxidized by dimethyl sulfoxide with acetic anhydride and give the lactone intermediate b; pyrrolo[2,1-f] [1,2,4]triazin-4-amine is brominated, and the amine group is protected by excess trimethylsilyl chloride. n-Butyllithium undergoes a halogen-lithium exchange reaction with the bromide at −78 °C (−108 °F) to yield the intermediate c. The intermediate b is then added to a solution containing intermediate c dropwise. After quenching the reaction in a weakly acidic aqueous solution, a mixture of 1: 1 anomers was obtained. It was then reacted with an excess of trimethylsilyl cyanide in dichloromethane at −78 °C (−108 °F) for 10 minutes. Trimethylsilyl triflate was added and reacts for one additional hour, and the mixture was quenched in an aqueous sodium hydrogen carbonate. A nitrile intermediate was obtained. The protective group, benzyl, was then removed with boron trichloride in dichloromethane at −20 °C (−4 °F). The excess of boron trichloride was quenched in a mixture of potassium carbonate and methanol. A benzyl-free intermediate was obtained. The isomers were then separated via reversed-phase HPLC. The optically pure compound and intermediate a are reacted with trimethyl phosphate and methylimidazole to obtain a diastereomer mixture of remdesivir. In the end, optically pure remdesivir can be obtained through chiral resolution methods.
Terminology
Remdesivir is the international nonproprietary name (INN)[43] while the development code name was GS-5734.[44]
Other animals
Remdesivir was shown in 2019 to have possible promise for treating feline infectious peritonitis caused by a coronavirus.[45] It has not been evaluated or approved by the Food and Drug Administration (FDA) for the treatment of feline coronavirus or feline infectious peritonitis but has been available since 2019 through websites and social media as an unregulated black market substance as confirmed by the UC Davis School of Veterinary Medicine.[46]
References
- Brunk D (7 February 2020). "Remdesivir Under Study as Treatment for Novel Coronavirus". Medscape. Retrieved 11 February 2020.
- Czech News Agency, "Did Czech scientists create the cure for coronavirus?", Aktuálně.cz, 5 February 2020.
- Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. (March 2016). "Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys". Nature. 531 (7594): 381–385. Bibcode:2016Natur.531..381W. doi:10.1038/nature17180. PMC 5551389. PMID 26934220.
- Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, et al. (March 2017). "GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses". Scientific Reports. 7: 43395. doi:10.1038/srep43395. PMID 28262699.
- "Coronavirus COVID-19 (SARS-CoV-2)". Johns Hopkins ABX Guide. Retrieved 17 April 2020.
Remdesivir: Likely the most promising drug.
- "Data on Gilead's remdesivir show no benefit for coronavirus patients". STAT. 23 April 2020. Retrieved 23 April 2020.
- "Remdesivir Clinical Trials". Gilead Sciences, Inc. Retrieved 20 April 2020.
- "7 Studies found for: Remdesivir & Recruiting, Not yet recruiting, Active, not recruiting, Completed, Enrolling by invitation Studies & COVID-19". ClinicalTrials.gov. U.S. National Library of Medicine. Retrieved 16 April 2020.
- "A Trial of Remdesivir in Adults With Severe COVID-19". ClinicalTrials.gov. 6 February 2020. Retrieved 19 April 2020.
- "A Trial of Remdesivir in Adults With Mild and Moderate COVID-19". ClinicalTrials.gov. 5 February 2020. Retrieved 19 April 2020.
- "Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment". ClinicalTrials.gov. 3 March 2020. Retrieved 19 April 2020.
- "Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)". ClinicalTrials.gov. 3 March 2020. Retrieved 19 April 2020.
- "Adaptive COVID-19 Treatment Trial (ACTT)". ClinicalTrials.gov. Retrieved 19 April 2020. Lay summary.
- "Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy)". ClinicalTrials.gov. 20 March 2020. Retrieved 19 April 2020.
- "Expanded Access Remdesivir (RDV; GS-5734)". ClinicalTrials.gov. 10 March 2020. Retrieved 19 April 2020.
- "Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the Treatment of SARS-CoV2 (CoV) Infection (COVID-19)". ClinicalTrials.gov. 27 March 2020. Retrieved 19 April 2020.
- "Multi-centre, adaptive, randomized trial of the safety and efficacy of treatments of COVID-19 in hospitalized adults (DisCoVeRy)". European Union Clinical Trials Register. Retrieved 19 April 2020.
- ""Solidarity" clinical trial for COVID-19 treatments". WHO. 3 March 2020. Retrieved 19 April 2020.
- "UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment". UN News. 18 March 2020.
- Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. (April 2020). "Compassionate Use of Remdesivir for Patients with Severe Covid-19". The New England Journal of Medicine. doi:10.1056/NEJMoa2007016. PMID 32275812.
- de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, et al. (March 2020). "Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection". Proc. Natl. Acad. Sci. U.S.A. 117 (12): 6771–6776. doi:10.1073/pnas.1922083117. PMC 7104368. PMID 32054787.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. (June 2017). "Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses". Science Translational Medicine. 9 (396): eaal3653. doi:10.1126/scitranslmed.aal3653. PMC 5567817. PMID 28659436.
- Joseph SS, Samuel M (31 January 2020). "Gilead working with China to test Ebola drug as new coronavirus treatment". Thomson Reuters. Archived from the original on 31 January 2020. Retrieved 31 January 2020.
- Munster V, Feldmann F, Williamson B, Van Doremalen N, Perez-Perez L, Schultz J, et al. (March 2020). "Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2". bioRxiv 10.1101/2020.03.21.001628.
- Williamson B, Feldmann F, Schwarz B, Meade-White K, Porter D, Schulz J, et al. (April 2020). "Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2". bioRxiv 10.1101/2020.04.15.043166.
- Barmann J. "Bay Area-Based Gilead Sees Potential Legal Conflict With China Over Its Coronavirus Drug". SFist. Impress Media. Archived from the original on 26 March 2020. Retrieved 22 March 2020.
- Antiviral Compound Provides Full Protection from Ebola Virus in Nonhuman Primates (PDF). United States Army Medical Research Institute of Infectious Diseases (USAMRIID) (Report). San Diego, California. 9 October 2015. Archived from the original (PDF) on 15 March 2020. Retrieved 15 March 2020.
- Preidt R (29 June 2017). "Experimental Drug Shows Promise Against Dangerous Viruses: Medicine worked in lab tests against germs that cause SARS and MERS infections". Archived from the original on 28 July 2017.
- Cihlar T (20 October 2015). "Discovery and Development of GS-5734, a Novel Nucleotide Prodrug with Broad Spectrum Anti-Filovirus Activity". FANG-WHO Workshop. Fort Detrick, MD: Gilead Sciences.
- Warren T, Jordan R, Lo M, Soloveva V, Ray A, Bannister R, et al. (Fall 2015). "Nucleotide Prodrug GS-5734 Is a Broad-Spectrum Filovirus Inhibitor That Provides Complete Therapeutic Protection Against the Development of Ebola Virus Disease (EVD) in Infected Non-human Primates". Open Forum Infectious Diseases. 2 (suppl 1): LB–2. doi:10.1093/ofid/ofv130.02.
- Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A, et al. (July 2016). "Late Ebola virus relapse causing meningoencephalitis: a case report". Lancet. 388 (10043): 498–503. doi:10.1016/S0140-6736(16)30386-5. PMC 4967715. PMID 27209148.
- Dwyer C (27 November 2018). "Ebola Treatment Trials Launched In Democratic Republic Of The Congo Amid Outbreak". National Public Radio. Retrieved 28 May 2019.
- McNeil DG (12 August 2019). "A Cure for Ebola? Two New Treatments Prove Highly Effective in Congo". The New York Times. Retrieved 13 August 2019.
- Molteni M (12 August 2019). "Ebola is Now Curable. Here's How The New Treatments Work". Wired. Retrieved 13 August 2019.
- "Data" (PDF). www.mzcr.cz. Retrieved 24 March 2020.
- Naftulin J (20 March 2020). "The FDA is allowing two drugs to be used for 'compassionate use' to treat the coronavirus. Here's what that means". Business Insider.
- Cerullo M (23 March 2020). "Gilead suspends emergency access to experimental coronavirus drug remdesivir". CBS News. Retrieved 23 March 2020.
- "Coronavirus COVID-19 (SARS-CoV-2)". Johns Hopkins ABX Guide. Retrieved 12 April 2020.
- Public Health Agency of Canada (11 April 2020). "Coronavirus disease (COVID-19): For health professionals". aem. Retrieved 12 April 2020.
Gilead is transitioning the provision of emergency access to remdesivir from individual compassionate use via Health Canada's Special Access Program requests to access through clinical trials.
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, et al. (March 2018). "Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease". mBio. 9 (2). doi:10.1128/mBio.00221-18. PMC 5844999. PMID 29511076.
- Tchesnokov EP, Feng JY, Porter DP, Götte M (April 2019). "Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir". Viruses. 11 (4): 326. doi:10.3390/v11040326. PMC 6520719. PMID 30987343.
- US 9724360, Chun BK, Clarke MO, Doerffler E, Hui HC, Jordan R, Mackman RL, Parrish JP, Ray AS, Siegel D, "Methods for treating Filoviridae virus infections", issued 19 July 2017, assigned to Gilead Sciences Inc.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information. 31 (3): 549. hdl:10665/330961.
- "Pipeline". Gilead. 27 February 2020. Retrieved 17 April 2020.
- Pedersen NC, Perron M, Bannasch M, Montgomery E, Murakami E, Liepnieks M, Liu H (April 2019). "Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis". Journal of Feline Medicine and Surgery. 21 (4): 271–281. doi:10.1177/1098612X19825701. PMC 6435921. PMID 30755068.
- Niels C. Pedersen (18 June 2019). "Black market production and sale of GS-441524 and GC376" (PDF). Feline Infectious Peritonitis Therapeutics/Clinical Trials Team. UC Davis. Retrieved 14 April 2020.
External links
- "Remdesivir". Drug Information Portal. U.S. National Library of Medicine.