Flavonoid
Flavonoids (or bioflavonoids) (from the Latin word flavus, meaning yellow, their color in nature) are a class of polyphenolic plant and fungus secondary metabolites.[1]


Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C).[1] This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature,[2][3] they can be classified into:
- flavonoids or bioflavonoids
- isoflavonoids, derived from 3-phenylchromen-4-one (3-phenyl-1,4-benzopyrone) structure
- neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure
The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins (flavones and flavonols).[1] This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non-ketone polyhydroxy polyphenol compounds, which are more specifically termed flavanoids. The three cycles or heterocycles in the flavonoid backbone are generally called ring A, B, and C. Ring A usually shows a phloroglucinol substitution pattern.
Biosynthesis
Functions of flavonoids in plants
Flavonoids are widely distributed in plants, fulfilling many functions. [1] Flavonoids are the most important plant pigments for flower coloration, producing yellow or red/blue pigmentation in petals designed to attract pollinator animals. In higher plants, flavonoids are involved in UV filtration, symbiotic nitrogen fixation and floral pigmentation. They may also act as chemical messengers, physiological regulators, and cell cycle inhibitors. Flavonoids secreted by the root of their host plant help Rhizobia in the infection stage of their symbiotic relationship with legumes like peas, beans, clover, and soy. Rhizobia living in soil are able to sense the flavonoids and this triggers the secretion of Nod factors, which in turn are recognized by the host plant and can lead to root hair deformation and several cellular responses such as ion fluxes and the formation of a root nodule. In addition, some flavonoids have inhibitory activity against organisms that cause plant diseases, e.g. Fusarium oxysporum.[4]
Subgroups
Over 5000 naturally occurring flavonoids have been characterized from various plants. They have been classified according to their chemical structure, and are usually subdivided into the following subgroups (for further reading see[5]):
Anthocyanidins
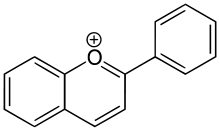
Anthocyanidins are the aglycones of anthocyanins; they use the flavylium (2-phenylchromenylium) ion skeleton.[1]
- Examples: Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin, Petunidin
Anthoxanthins
Anthoxanthins are divided into two groups:[6]
| Group | Skeleton | Examples | |||
|---|---|---|---|---|---|
| Description | Functional groups | Structural formula | |||
| 3-hydroxyl | 2,3-dihydro | ||||
| Flavone | 2-phenylchromen-4-one | ✗ | ✗ |  |
Luteolin, Apigenin, Tangeritin |
| Flavonol or 3-hydroxyflavone |
3-hydroxy-2-phenylchromen-4-one | ✓ | ✗ |  |
Quercetin, Kaempferol, Myricetin, Fisetin, Galangin, Isorhamnetin, Pachypodol, Rhamnazin, Pyranoflavonols, Furanoflavonols, |
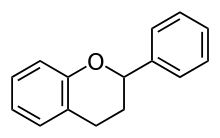
Flavanones
Flavanones
| Group | Skeleton | Examples | |||
|---|---|---|---|---|---|
| Description | Functional groups | Structural formula | |||
| 3-hydroxyl | 2,3-dihydro | ||||
| Flavanone | 2,3-dihydro-2-phenylchromen-4-one | ✗ | ✓ | 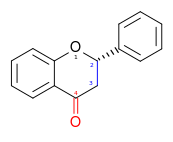 |
Hesperetin, Naringenin, Eriodictyol, Homoeriodictyol |
Flavanonols
Flavanonols
| Group | Skeleton | Examples | |||
|---|---|---|---|---|---|
| Description | Functional groups | Structural formula | |||
| 3-hydroxyl | 2,3-dihydro | ||||
| Flavanonol or 3-Hydroxyflavanone or 2,3-dihydroflavonol |
3-hydroxy-2,3-dihydro-2-phenylchromen-4-one | ✓ | ✓ |  |
Taxifolin (or Dihydroquercetin), Dihydrokaempferol |
Flavans
Include flavan-3-ols (flavanols), flavan-4-ols and flavan-3,4-diols.
| Skeleton | Name |
|---|---|
 |
Flavan-3-ol (flavanol) |
 |
Flavan-4-ol |
 |
Flavan-3,4-diol (leucoanthocyanidin) |
- Flavan-3-ols (flavanols)
- Flavan-3-ols use the 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton
- Examples: Catechin (C), Gallocatechin (GC), Catechin 3-gallate (Cg), Gallocatechin 3-gallate (GCg), Epicatechins (Epicatechin (EC)), Epigallocatechin (EGC), Epicatechin 3-gallate (ECg), Epigallocatechin 3-gallate (EGCg)
- Theaflavin
- Examples: Theaflavin-3-gallate, Theaflavin-3'-gallate, Theaflavin-3,3'-digallate
- Thearubigin
- Proanthocyanidins are dimers, trimers, oligomers, or polymers of the flavanols
Isoflavonoids
- Isoflavonoids
- Isoflavones use the 3-phenylchromen-4-one skeleton (with no hydroxyl group substitution on carbon at position 2)
- Examples: Genistein, Daidzein, Glycitein
- Isoflavanes
- Isoflavandiols
- Isoflavenes
- Coumestans
- Pterocarpans
Dietary sources


Flavonoids (specifically flavanoids such as the catechins) are "the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants".[1][7] Flavonols, the original bioflavonoids such as quercetin, are also found ubiquitously, but in lesser quantities. The widespread distribution of flavonoids, their variety and their relatively low toxicity compared to other active plant compounds (for instance alkaloids) mean that many animals, including humans, ingest significant quantities in their diet.[1] Foods with a high flavonoid content include parsley,[8] onions,[8] blueberries and other berries,[8] black tea,[8] green tea and oolong tea,[8] bananas, all citrus fruits, Ginkgo biloba, red wine, sea-buckthorns, buckwheat,[9] and dark chocolate (with a cocoa content of 70% or greater). Further information on dietary sources of flavonoids can be obtained from the US Department of Agriculture flavonoid database.[8]
Blueberries
Blueberries are a dietary source of anthocyanidins.[8][10]
Black tea
Black tea is a rich source of dietary flavan-3-ols.[8]
Citrus
The citrus flavonoids include hesperidin (a glycoside of the flavanone hesperetin), quercitrin, rutin (two glycosides of the flavonol quercetin), and the flavone tangeritin.
Wine
Dietary intake
_in_the_European_Union.png)
Food composition data for flavonoids were provided by the USDA database on flavonoids.[8] In the United States NHANES survey, mean flavonoid intake was 190 mg/d in adults, with flavan-3-ols as the main contributor.[18] In the European Union, based on data from EFSA, mean flavonoid intake was 140 mg/d, although there were considerable differences between individual countries.[17]
_in_the_European_Union.png)
The main type of flavonoids consumed in the EU and USA were flavan-3-ols, mainly from tea, while intake of other flavonoids was considerably lower.[17][18]
Research
Though there is ongoing research into the potential health benefits of individual flavonoids, neither the Food and Drug Administration (FDA) nor the European Food Safety Authority (EFSA) has approved any health claim for flavonoids or approved any flavonoids as pharmaceutical drugs.[1][19][20][21] Moreover, several companies have been cautioned by the FDA over misleading health claims.[22][23][24][25]. A web resource provides physicochemical properties and comprehensive literature of flavonoids.[26][27]
In vitro
Laboratory studies indicate that flavonoids have effects on isolated cells or cell cultures in vitro, but there is no such evidence from human clinical research.[1]
Antioxidant
Flavonoids are poorly absorbed in the human body (less than 5%), then are quickly metabolized into smaller fragments with unknown properties, and rapidly excreted.[21][28][29] Flavonoids have negligible antioxidant activity in the body, and the increase in antioxidant capacity of blood seen after consumption of flavonoid-rich foods is not caused directly by flavonoids, but is due to production of uric acid resulting from flavonoid depolymerization and excretion.[30]
Inflammation
Inflammation has been implicated as a possible origin of numerous local and systemic diseases, such as cancer,[31] cardiovascular disorders,[32] diabetes mellitus,[33] and celiac disease.[34]
Preliminary studies indicate that flavonoids may affect anti-inflammatory mechanisms via their ability to inhibit reactive oxygen or nitrogen compounds.[35] Flavonoids have also been proposed to inhibit the pro-inflammatory activity of enzymes involved in free radical production, such as cyclooxygenase, lipoxygenase or inducible nitric oxide synthase,[35][36] and to modify intracellular signaling pathways in immune cells,[35] or in brain cells after a stroke.[37]
Procyanidins, a class of flavonoids, have been shown in preliminary research to have anti-inflammatory mechanisms including modulation of the arachidonic acid pathway, inhibition of gene transcription, expression and activity of inflammatory enzymes, as well as secretion of anti-inflammatory mediators.[38]
Cancer
Clinical studies investigating the relationship between flavonoid consumption and cancer prevention/development are conflicting for most types of cancer, probably because most human studies have weak designs, such as a small sample size.[1][39] Two apparent exceptions are gastric carcinoma and smoking-related cancers. Dietary flavonoid intake is associated with reduced gastric carcinoma risk in women,[40] and reduced aerodigestive tract cancer risk in smokers.[41]
Cardiovascular diseases
Among the most intensively studied of general human disorders possibly affected by dietary flavonoids, only preliminary cardiovascular disease research has been investigated:[42][43][44][45][46]
However, population-based studies have failed to show a strong beneficial effect,[1][47] which might be due to the considerably lower intake in the habitual diet of those investigated.
Antibacterial
The plants with flavonoids as their major constituents can inhibit Helicobacter pylori infection and use as anti peptic ulcer disease.[48]
Synthesis, detection, quantification, and semi-synthetic alterations
Color spectrum
Flavonoid synthesis in plants is induced by light color spectrums at both high and low energy radiations. Low energy radiations are accepted by phytochrome, while high energy radiations are accepted by carotenoids, flavins, cryptochromes in addition to phytochromes. The photomorphogenic process of phytochrome-mediated flavonoid biosynthesis has been observed in Amaranthus, barley, maize, Sorghum and turnip. Red light promotes flavonoid synthesis.[49]
Availability through microorganisms
Several recent research articles have demonstrated the efficient production of flavonoid molecules from genetically engineered microorganisms.[50][51][52]
Tests for detection
- Shinoda test
Four pieces of magnesium filings are added to the ethanolic extract followed by few drops of concentrated hydrochloric acid. A pink or red colour indicates the presence of flavonoid.[53] Colours varying from orange to red indicated flavones, red to crimson indicated flavonoids, crimson to magenta indicated flavonones.
- Sodium hydroxide test
About 5 mg of the compound is dissolved in water, warmed and filtered. 10% aqueous sodium hydroxide is added to 2 ml of this solution. This produces a yellow coloration. A change in color from yellow to colorless on addition of dilute hydrochloric acid is an indication for the presence of flavonoids.[54]
- p-Dimethylaminocinnamaldehyde test
A colorimetric assay based upon the reaction of A-rings with the chromogen p-dimethylaminocinnamaldehyde (DMACA) has been developed for flavanoids in beer that can be compared with the vanillin procedure.[55]
Quantification
Lamaison and Carnet have designed a test for the determination of the total flavonoid content of a sample (AlCI3 method). After proper mixing of the sample and the reagent, the mixture is incubated for 10 minutes at ambient temperature and the absorbance of the solution is read at 440 nm. Flavonoid content is expressed in mg/g of quercetin.[56]
Semi-synthetic alterations
Immobilized Candida antarctica lipase can be used to catalyze the regioselective acylation of flavonoids.[57]
See also
- Phytochemical
- List of antioxidants in food
- List of phytochemicals in food
- Phytochemistry
- Secondary metabolites
- Homoisoflavonoids, related chemicals with a 16 carbons skeleton
References
- "Flavonoids". Linus Pauling Institute, Oregon State University, Corvallis, OR. 2016. Retrieved 22 April 2020.
- McNaught, Alan D; Wilkinson, Andrew; IUPAC (1997), IUPAC Compendium of Chemical Terminology (2 ed.), Oxford: Blackwell Scientific, doi:10.1351/goldbook.F02424, ISBN 978-0-9678550-9-7
- "Flavonoids (isoflavonoids and neoflavonoids)". The Gold Book. 2009. doi:10.1351/goldbook. ISBN 978-0-9678550-9-7. Retrieved 16 September 2012.
- Galeotti, F; Barile, E; Curir, P; Dolci, M; Lanzotti, V (2008). "Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity". Phytochemistry Letters. 1: 44–48. doi:10.1016/j.phytol.2007.10.001.
- Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (October 2007). "Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health". Biotechnology Journal. 2 (10): 1214–34. doi:10.1002/biot.200700084. PMID 17935117.
- Isolation of a UDP-glucose: Flavonoid 5-O-glucosyltransferase gene and expression analysis of anthocyanin biosynthetic genes in herbaceous peony (Paeonia lactiflora Pall.). Da Qiu Zhao, Chen Xia Han, Jin Tao Ge and Jun Tao, Electronic Journal of Biotechnology, 15 November 2012, Volume 15, Number 6, doi:10.2225/vol15-issue6-fulltext-7
- Spencer JP (2008). "Flavonoids: modulators of brain function?". British Journal of Nutrition. 99 (E-S1): ES60–77. doi:10.1017/S0007114508965776. PMID 18503736.
- USDA’s Database on the Flavonoid Content
- Oomah, B. Dave; Mazza, Giuseppe (1996). "Flavonoids and Antioxidative Activities in Buckwheat". Journal of Agricultural and Food Chemistry. 44 (7): 1746–1750. doi:10.1021/jf9508357.
- Ayoub M, de Camargo AC, Shahidi F (2016). "Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals". Food Chemistry. 197 (Part A): 221–232. doi:10.1016/j.foodchem.2015.10.107. PMID 26616944.
- The Lancet (2007). "The devil in the dark chocolate". Lancet. 370 (9605): 2070. doi:10.1016/S0140-6736(07)61873-X. PMID 18156011.
- Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A (2003). "Plasma antioxidants from chocolate" (PDF). Nature. 424 (6952): 1013. Bibcode:2003Natur.424.1013S. doi:10.1038/4241013a. PMID 12944955.
- Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A (2003). "Nutrition: milk and absorption of dietary flavanols" (PDF). Nature. 424 (6952): 1013. Bibcode:2003Natur.424.1013S. doi:10.1038/4241013a. PMID 12944955.
- Roura E, et al. (2007). "Milk Does Not Affect the Bioavailability of Cocoa Powder Flavonoid in Healthy Human" (PDF). Ann Nutr Metab. 51 (6): 493–498. doi:10.1159/000111473. PMID 18032884.
- de Camargo AC, Regitano-d'Arce MA, Gallo CR, Shahidi F (2015). "Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin". Journal of Functional Foods. 12: 129–143. doi:10.1016/j.jff.2014.10.034.
- Chukwumah Y, Walker LT, Verghese M (2009). "Peanut skin color: a biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars". Int J Mol Sci. 10 (11): 4941–52. doi:10.3390/ijms10114941. PMC 2808014. PMID 20087468.
- Vogiatzoglou, A; Mulligan, A. A.; Lentjes, M. A.; Luben, R. N.; Spencer, J. P.; Schroeter, H; Khaw, K. T.; Kuhnle, G. G. (2015). "Flavonoid intake in European adults (18 to 64 years)". PLoS ONE. 10 (5): e0128132. doi:10.1371/journal.pone.0128132. PMC 4444122. PMID 26010916.
- Chun, O. K.; Chung, S. J.; Song, W. O. (2007). "Estimated dietary flavonoid intake and major food sources of U.S. Adults". The Journal of Nutrition. 137 (5): 1244–52. doi:10.1093/jn/137.5.1244. PMID 17449588.
- "FDA approved drug products". US Food and Drug Administration. Retrieved 8 November 2013.
- "Health Claims Meeting Significant Scientific Agreement". US Food and Drug Administration. Retrieved 8 November 2013.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2010). "Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/20061". EFSA Journal. 8 (2): 1489. doi:10.2903/j.efsa.2010.1489.
- "Inspections, Compliance, Enforcement, and Criminal Investigations (Flavonoid Sciences)". US Food and Drug Administration. Retrieved 8 November 2013.
- "Inspections, Compliance, Enforcement, and Criminal Investigations (Unilever, Inc.)". US Food and Drug Administration. Retrieved 25 October 2013.
- "Lipton green tea is a drug". NutraIngredients-USA.com. Retrieved 25 October 2013.
- "Fruits Are Good for Your Health? Not So Fast: FDA Stops Companies From Making Health Claims About Foods". TheDailyGreen.com. Retrieved 25 October 2013.
- Kolte BS, Londhe SR, Bagul KT, Pawnikar SP, Goundge MB, Gacche RN, Meshram RJ (2019). "FlavoDb: a web-based chemical repository of flavonoid compounds". 3 Biotech. 9 (11): 431. doi:10.1007/s13205-019-1962-7.
- "FlavoDB". Bioinformatics Centre, Savitribai Phule Pune University, Pune, India. Retrieved 7 November 2019.
- Lotito SB, Frei B (2006). "Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon?". Free Radic. Biol. Med. 41 (12): 1727–46. doi:10.1016/j.freeradbiomed.2006.04.033. PMID 17157175.
- Williams RJ, Spencer JP, Rice-Evans C (2004). "Flavonoids: antioxidants or signalling molecules?". Free Radical Biology & Medicine. 36 (7): 838–49. doi:10.1016/j.freeradbiomed.2004.01.001. PMID 15019969.
- Stauth D (5 March 2007). "Studies force new view on biology of flavonoids". EurekAlert!, Adapted from a news release issued by Oregon State University.
- Ravishankar D, Rajora AK, Greco F, Osborn HM (2013). "Flavonoids as prospective compounds for anti-cancer therapy". The International Journal of Biochemistry & Cell Biology. 45 (12): 2821–2831. doi:10.1016/j.biocel.2013.10.004. PMID 24128857.
- Manach C, Mazur A, Scalbert A (2005). "Polyphenols and prevention of cardiovascular diseases". Current Opinion in Lipidology. 16 (1): 77–84. doi:10.1097/00041433-200502000-00013. PMID 15650567.
- Babu PV, Liu D, Gilbert ER (2013). "Recent advances in understanding the anti-diabetic actions of dietary flavonoids". The Journal of Nutritional Biochemistry. 24 (11): 1777–1789. doi:10.1016/j.jnutbio.2013.06.003. PMC 3821977. PMID 24029069.
- Ferretti G, Bacchetti T, Masciangelo S, Saturni L (2012). "Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach". Nutrients. 4 (12): 243–257. doi:10.3390/nu4040243. PMC 3347005. PMID 22606367.
- Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, Bei R (2012). "The effects of dietary flavonoids on the regulation of redox inflammatory networks". Frontiers in Bioscience. 17 (7): 2396–2418. doi:10.2741/4061. PMID 22652788.
- Gomes A, Couto D, Alves A, Dias I, Freitas M, Porto G, Duarte JA, Fernandes E (2012). "Trihydroxyflavones with antioxidant and anti-inflammatory efficacy". BioFactors. 38 (5): 378–386. doi:10.1002/biof.1033. PMID 22806885.
- Chang CF, Cho S, Wang J (Apr 2014). "(-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways". Ann Clin Transl Neurol. 1 (4): 258–271. doi:10.1002/acn3.54. PMC 3984761. PMID 24741667.
- Martinez-Micaelo N, González-Abuín N, Ardèvol A, Pinent M, Blay MT (2012). "Procyanidins and inflammation: Molecular targets and health implications". BioFactors. 38 (4): 257–265. doi:10.1002/biof.1019. PMID 22505223.
- Romagnolo DF, Selmin OI (2012). "Flavonoids and cancer prevention: a review of the evidence". J Nutr Gerontol Geriatr. 31 (3): 206–38. doi:10.1080/21551197.2012.702534. PMID 22888839.
- González CA, Sala N, Rokkas T (2013). "Gastric cancer: epidemiologic aspects". Helicobacter. 18 (Supplement 1): 34–38. doi:10.1111/hel.12082. PMID 24011243.
- Woo HD, Kim J (2013). "Dietary flavonoid intake and smoking-related cancer risk: a meta-analysis". PLoS ONE. 8 (9): e75604. Bibcode:2013PLoSO...875604W. doi:10.1371/journal.pone.0075604. PMC 3777962. PMID 24069431.
- Higdon, J; Drake, V; Frei, B (March 2009). "Non-Antioxidant Roles for Dietary Flavonoids: Reviewing the relevance to cancer and cardiovascular diseases". Nutraceuticals World. Rodman Media. Retrieved 24 November 2013.
- van Dam RM, Naidoo N, Landberg R (2013). "Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases". Current Opinion in Lipidology. 24 (1): 25–33. doi:10.1097/MOL.0b013e32835bcdff. PMID 23254472.
- Tangney CC, Rasmussen HE (2013). "Polyphenols, Inflammation, and Cardiovascular Disease". Current Atherosclerosis Reports. 15 (5): 324. doi:10.1007/s11883-013-0324-x. PMC 3651847. PMID 23512608.
- Siasos G, Tousoulis D, Tsigkou V, Kokkou E, Oikonomou E, Vavuranakis M, Basdra EK, Papavassiliou AG, Stefanadis C (2013). "Flavonoids in atherosclerosis: An overview of their mechanisms of action". Current Medicinal Chemistry. 20 (21): 2641–2660. doi:10.2174/0929867311320210003. PMID 23627935.
- Cappello, AR, Dolce V, Iacopetta D, Martello M, Fiorillo M, Curcio R, Muto L, Dhanyalayam D. (2015). "Bergamot (Citrus bergamia Risso) Flavonoids and Their Potential Benefits in Human Hyperlipidemia and Atherosclerosis: an Overview". Mini-Reviews in Medicinal Chemistry. 16 (8): 1–11. doi:10.2174/1389557515666150709110222. PMID 26156545.
- Wang X; Ouyang YY; Liu J; Zhao G (January 2014). "Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies". The British Journal of Nutrition. 111 (1): 1–11. doi:10.1017/S000711451300278X. PMID 23953879.
- Ardalani, Hamidreza; Hadipanah, Amin; Sahebkar, Amirhossein (27 December 2019). "Medicinal plants in the treatment of peptic ulcer disease: A review". Mini-Reviews in Medicinal Chemistry. 20. doi:10.2174/1389557520666191227151939.
- Sinha, Rajiv Kumar (2004-01-01). Modern Plant Physiology. CRC Press. p. 457. ISBN 9780849317149.
- Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S (May 2003). "Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster". Appl. Environ. Microbiol. 69 (5): 2699–706. doi:10.1128/AEM.69.5.2699-2706.2003. PMC 154558. PMID 12732539.
- Trantas E, Panopoulos N, Ververidis F (2009). "Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae". Metabolic Engineering. 11 (6): 355–366. doi:10.1016/j.ymben.2009.07.004. PMID 19631278.
- Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (2007). "Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part II: Reconstruction of multienzyme pathways in plants and microbes". Biotechnology Journal. 2 (10): 1235–49. doi:10.1002/biot.200700184. PMID 17935118.
- Yisa, Jonathan (2009). "Phytochemical Analysis and Antimicrobial Activity Of Scoparia Dulcis and Nymphaea Lotus". Australian Journal of Basic and Applied Sciences. 3 (4): 3975–3979.
- Bello IA, Ndukwe GI, Audu OT, Habila JD (2011). "A bioactive flavonoid from Pavetta crassipes K. Schum". Organic and Medicinal Chemistry Letters. 1 (1): 14. doi:10.1186/2191-2858-1-14. PMC 3305906. PMID 22373191.
- A new colourimetric assay for flavonoids in pilsner beers. Jan A. Delcour and Didier Janssens de Varebeke, Journal of the Institute of Brewing, January–February 1985, Volume 91, Issue 1, pages 37–40, doi:10.1002/j.2050-0416.1985.tb04303.x
- Lamaison, JL; Carnet, A (1991). "Teneurs en principaux flavonoides des fleurs de Cratageus monogyna Jacq et de Cratageus Laevigata (Poiret D.C) en Fonction de la vegetation". Plantes Medicinales Phytotherapie. 25: 12–16.
- Passicos E, Santarelli X, Coulon D (2004). "Regioselective acylation of flavonoids catalyzed by immobilized Candida antarctica lipase under reduced pressure". Biotechnol. Lett. 26 (13): 1073–1076. doi:10.1023/B:BILE.0000032967.23282.15. PMID 15218382.
Further reading
- Andersen, Ø.M. / Markham, K.R. (2006). Flavonoids: Chemistry, Biochemistry and Applications. CRC Press. ISBN 978-0-8493-2021-7
- Grotewold, Erich (2007). The Science of Flavonoids. Springer. ISBN 978-0-387-74550-3
- Comparative Biochemistry of the Flavonoids, by J.B. Harborne, 1967 (Google Books)
- The systematic identification of flavonoids, by T.J. Mabry, K.R. Markham and M.B. Thomas, 1970, doi:10.1016/0022-2860(71)87109-0
| Wikimedia Commons has media related to Flavonoids. |