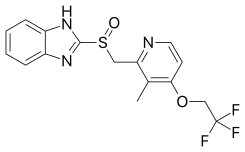
Lansoprazole
Lansoprazole, sold under the brand name Prevacid among others, is a medication which reduces stomach acid.[1] It is used to treat peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome.[2] Effectiveness is similar to other proton pump inhibitors (PPIs).[3] It is taken by mouth.[1] Onset is over a few hours and effects last up to a couple of days.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /lænˈsoʊprəzoʊl/ lan-SOH-prə-zohl |
| Trade names | Prevacid, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695020 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, IV |
| Drug class | Proton pump inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% or more |
| Protein binding | 97% |
| Metabolism | Liver (CYP3A4- and CYP2C19-mediated) |
| Elimination half-life | 1.0–1.5 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.173.220 |
| Chemical and physical data | |
| Formula | C16H14F3N3O2S |
| Molar mass | 369.363 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Common side effects include constipation, abdominal pain, and nausea.[1][4] Serious side effects may include osteoporosis, low blood magnesium, Clostridium difficile infection, and pneumonia.[1][4] Use in pregnancy and breastfeeding is of unclear safety.[5] It works by blocking H+/K+-ATPase in the parietal cells of the stomach.[1]
Lansoprazole was patented in 1984 and came into medical use in 1992.[6] It is available as a generic medication.[2] A one month supply, in the United Kingdom, costs the NHS less than £5, as of 2019.[2] In the United States, the wholesale cost of this amount is about $5.40, as of 2019.[7] In 2017, it was the 188th most commonly prescribed medication in the United States, with more than three million prescriptions.[8][9]
Medical uses
Lansoprazole is used for treatment of:[4]
- Ulcers of the stomach and duodenum, and NSAID-induced ulcers
- Helicobacter pylori infection, alongside antibiotics (adjunctive treatment), treatment to kill H. pylori causing ulcers or other problems involves using two other drugs besides lansoprazole known as "triple therapy", and involves taking twice daily for 10 or 14 days lansoprazole, amoxicillin, and clarithromycin
- Gastroesophageal reflux disease
- Zollinger-Ellison syndrome[10]
There is no good evidence that it works better than other PPIs.[3]
Side effects
Side effects of PPIs in general[11] and lansoprazole in particular[12] may include:[4]
- Common: diarrhea, abdominal pain[13]
- Infrequent: dry mouth, insomnia, drowsiness, blurred vision, rash, pruritus
- Rarely and very rarely: taste disturbance, liver dysfunction, peripheral oedema, hypersensitivity reactions (including bronchospasm, urinary, angioedema, anaphylaxis), photosensitivity, fever, sweating, depression, interstitial nephritis, blood disorders (including leukopenia, leukocytosis, pancytopenia, thrombocytopenia), arthralgia, myalgia, skin reactions[14] including (erythroderma[15] Stevens–Johnson syndrome, toxic epidermal necrolysis, bullous eruption)
PPIs may be associated with a greater risk of hip fractures and Clostridium difficile-associated diarrhea.[4]:22
Interactions
Lansoprazole interacts with several other drugs, either due to its own nature or as a PPI.[16]
- PPIs reduce absorption of antifungals (itraconazole and ketoconazole) [17] and possibly increase digoxin in plasma
- Increases plasma concentrations of cilostazol (risk of toxicity)
Lansoprazole possibly interacts with, among other drugs:
Chemistry
It is a racemic 1:1 mixture of the enantiomers dexlansoprazole and levolansoprazole.[18] Dexlansoprazole is an enantiomerically pure active ingredient of a commercial drug as a result of the enantiomeric shift. Lansoprazole's plasma elimination half-life (1.5 h) is not proportional to the duration of the drug's effects to the person (i.e. gastric acid suppression).[19]
History
Lansoprazole was originally synthesized at Takeda and was given the development name AG 1749.[20] Takeda patented it in 1984 and the drug launched in 1991.[21] In the United States, it was approved for medical use in 1995.[22]
Society and culture

Patents
The lansoprazole molecule is off-patent and so generic drugs are available under many brand names in many countries;[23] there are patents covering some formulations in effect as of 2015.[24] Patent protection expired on 10 November 2009.[25][26]
Availability
Since 2009, lansoprazole has been available over the counter (OTC) in the U.S. as Prevacid 24HR[27][28] and as Lansoprazole 24HR.[29] In Australia, it is marketed by Pfizer as Zoton.
Research
In vitro experiments have shown that lansoprazole binds to the pathogenic form of tau protein.[30] As of 2015 laboratory studies were underway on analogs of lansoprazole to explore their use as potential PET imaging agents for diagnosing tauopathies including Alzheimer's disease.[30]
References
- "Lansoprazole Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 79–80. ISBN 9780857113382.
- "[99] Comparative effectiveness of proton pump inhibitors | Therapeutics Initiative". 28 June 2016. Retrieved 14 July 2016.
- "Lansoprazole capsule, delayed release pellets". DailyMed. 11 October 2016. Retrieved 31 December 2019.
- "Lansoprazole Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 445. ISBN 9783527607495.
- "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Lansoprazole - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Hirschowitz BI, Mohnen J, Shaw S (August 1996). "Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrome". Aliment. Pharmacol. Ther. 10 (4): 507–22. doi:10.1046/j.1365-2036.1996.10152000.x. PMID 8853754.
- British National Formulary (Free registration required) 1.3.5 Proton pump inhibitors
- British National Formulary (Free registration required) Lansoprazole
- "Prevacid (Lansoprazole) Drug Information: Side Effects and Drug Interactions - Prescribing Information at RxList". RxList. Retrieved 9 February 2016.
- K C Singhal & S Z Rahman, Lansoprazole Induced Adverse Effects on the Skin, Indian Medical Gazette, July 2001, Vol. CXXXV. N0. 7: 223-225
- Sterry W, Assaf C (2007). "Erythroderma". In Bolognia JL (ed.). Dermatology. St. Louis: Mosby. p. 154. ISBN 978-1-4160-2999-1..
- British National Formulary (Free registration required) Lansoprazole interactions
- Piscitelli, S. C.; Goss, T. F.; Wilton, J. H.; d'Andrea, D. T.; Goldstein, H; Schentag, J. J. (1991). "Effects of ranitidine and sucralfate on ketoconazole bioavailability". Antimicrobial Agents and Chemotherapy. 35 (9): 1765–1771. doi:10.1128/aac.35.9.1765. PMC 245265. PMID 1952845.
- "Pharmacy Benefit Update". Retrieved 2 July 2014.
- "Prevacid Pharmacology, Pharmacokinetics, Studies, Metabolism". RxList.com. 2007. Archived from the original on 16 August 2000. Retrieved 14 April 2007.
- Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 102. ISBN 9783527607495.
- Chorghade, Mukund S. (2006). Drug Discovery and Development, Volume 1: Drug Discovery. John Wiley & Sons. p. 201. ISBN 9780471780090.
- Mosby's Drug Consult: Lansoprazole
- drugs.com International availability of lansoprazole Page accessed 3 February 2015
- drugs.com Generic lansoprazole Page accessed 3 February 2015
- "Prevacid Drug Profile". Drugpatentwatch.com. Retrieved 30 April 2020.
- Teva to release Prevacid version when patent expires
- "Prevacid 24 HR- lansoprazole capsule, delayed release". DailyMed. 7 August 2019. Retrieved 31 December 2019.
- "Prevacid 24 HR- lansoprazole capsule, delayed release". DailyMed. 11 December 2019. Retrieved 31 December 2019.
- "Lansoprazole 24 HR- lansoprazole capsule, delayed release". DailyMed. 21 December 2017. Retrieved 31 December 2019.
- Villemagne, VL; Fodero-Tavoletti, MT; Masters, CL; Rowe, CC (January 2015). "Tau imaging: early progress and future directions". The Lancet. Neurology. 14 (1): 114–24. doi:10.1016/s1474-4422(14)70252-2. PMID 25496902.
External links
- "Lansoprazole". Drug Information Portal. U.S. National Library of Medicine.