Alginic acid
Alginic acid, also called algin, is a polysaccharide distributed widely in the cell walls of brown algae that is hydrophilic and forms a viscous gum when hydrated. With metals such as sodium and calcium, its salts are known as alginates. It is a significant component of the biofilms produced by the bacterium Pseudomonas aeruginosa, a major pathogen found in the lungs of some people who have cystic fibrosis.[1] The biofilm and P. aeruginosa have a high resistance to antibiotics,[2] and are susceptible to inhibition by macrophages.[3] Its colour ranges from white to yellowish-brown. It is sold in filamentous, granular, or powdered forms.
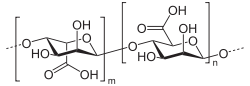 | |
| Names | |
|---|---|
| Other names
Alginic acid; E400; [D-ManA(β1→4)L-GulA(α1→4)]n | |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.029.697 |
| EC Number |
|
| E number | E400 (thickeners, ...) |
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| (C6H8O6)n | |
| Molar mass | 10,000 – 600,000 |
| Appearance | white to yellow, fibrous powder |
| Density | 1.601 g/cm3 |
| Acidity (pKa) | 1.5–3.5 |
| Pharmacology | |
| A02BX13 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

Structure
Alginic acid is a linear copolymer with homopolymeric blocks of (1-4)-linked β-D-mannuronate (M) and its C-5 epimer α-L-guluronate (G) residues, respectively, covalently linked together in different sequences or blocks. The monomers may appear in homopolymeric blocks of consecutive G-residues (G-blocks), consecutive M-residues (M-blocks) or alternating M and G-residues (MG-blocks).
Forms
Alginates are refined from brown seaweeds. A wide variety of brown seaweeds of the class Phaeophyceae are harvested throughout the world to be converted into the raw material commonly known as sodium alginate. Sodium alginate has a wide use across a wide variety of industries including food, textile printing, and pharmaceutical. Dental impression material uses alginate as its means of gelling. Alginate is safe as an ingredient in manufactured foods.[4]
Seaweeds may be classified into three broad groups based on pigmentation: brown, red, and green. These broad groups are the Phaeophyceae, Rhodophyceae, and Chlorophyceae, respectively. Brown seaweeds usually are large, and range from the giant kelp Macrocystis pyrifera that is often 20 m long, to thick, leather-like seaweeds from 2–4 m long, to smaller species 30–60 cm long. None of the usual seaweeds for alginate production are cultivated. They cannot be grown by vegetative means, but must go through a reproductive cycle involving an alternation of generations. This makes cultivated brown seaweeds too expensive when compared to the costs of harvesting and transporting wild seaweeds. The only exception is for Laminaria japonica, which is cultivated in China for food and its surplus material is diverted to the alginate industry in China.
Alginates from different species of brown seaweed often have variations in their chemical structure, resulting in different physical properties. For example, some may yield an alginate that gives a strong gel, another a weaker gel, some may readily give a cream or white alginate, while others are difficult to gel and are best used for technical applications where color does not matter.[5]
Commercial varieties of alginate are extracted from seaweed, including the giant kelp Macrocystis pyrifera, Ascophyllum nodosum, and various types of Laminaria. It also is produced by two bacterial genera Pseudomonas and Azotobacter, which played a major role in the unravelling of its biosynthesis pathway. Bacterial alginates are useful for the production of micro- or nanostructures suitable for medical applications.[6]
Sodium alginate is the sodium salt of alginic acid. Its empirical formula is NaC6H7O6. Sodium alginate is a gum, extracted from the cell walls of brown algae.
Potassium alginate is a chemical compound that is the potassium salt of alginic acid. It is an extract of seaweed. Its empirical chemical formula is KC6H7O6.
Calcium alginate, made from sodium alginate from which the sodium ion has been removed and replaced with calcium, has the chemical formula C12H14CaO12.
Production
The processes for the manufacture of sodium alginate from brown seaweeds fall into two categories: 1) Calcium alginate method and, 2) Alginic acid method. The chemistry of the processes used to extract sodium alginate from brown seaweeds is relatively simple. The difficulties of the processes arise from the physical separations that are required, such as the need to filter slimy residues from viscous solutions or to separate gelatinous precipitates that hold large amounts of liquid within the structure and thereby resist filtration and centrifugation.[7]
Uses
Alginate absorbs water quickly, which makes it useful as an additive in dehydrated products such as slimming aids, and in the manufacture of paper and textiles. It also is used for waterproofing and fireproofing fabrics, in the food industry as a thickening agent for drinks, ice cream, cosmetics, and as a gelling agent for jellies.
Alginate is used as an ingredient in various pharmaceutical preparations, such as Gaviscon, in which it combines with bicarbonate to inhibit reflux. Sodium alginate is used as an impression-making material in dentistry, prosthetics, lifecasting, and for creating positives for small-scale casting.
Sodium alginate is used in reactive dye printing and as a thickener for reactive dyes in textile screen-printing. Alginates do not react with these dyes and wash out easily, unlike starch-based thickeners.
As a material for micro-encapsulation.[8]
Calcium alginate is used in different types of medical products, including skin wound dressings to promote healing,[9][10] and may be removed with less pain than conventional dressings.
Alginate hydrogels
Alginate may be used in a hydrogel consisting of microparticles or bulk gels combined with nerve growth factor in bioengineering research to stimulate brain tissue for possible regeneration.[11] In research on bone reconstruction, alginate composites have favorable properties encouraging regeneration, such as improved porosity, cell proliferation, and mechanical strength, among other factors.[12]
See also
- Hyaluronic acid: a polysaccharide in animals.
- Agar
References
- Davies, JC (2002). "Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence". Paediatric Respiratory Reviews. 3 (2): 128–34. doi:10.1016/S1526-0550(02)00003-3. ISSN 1526-0542. PMID 12297059.
- Boyd, A; Chakrabarty, AM (1995). "Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide". Journal of Industrial Microbiology. 15 (3): 162–8. doi:10.1007/BF01569821. ISSN 0169-4146. PMID 8519473.
- Leid, JG; Willson, CJ; Shirtliff, ME; Hassett, DJ; Parsek, MR; Jeffers, AK (1 November 2005). "The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing" (PDF). Journal of Immunology. 175 (11): 7512–8. doi:10.4049/jimmunol.175.11.7512. ISSN 0022-1767. PMID 16301659.
- "Alginates" (PDF). Agricultural Marketing Service, US Department of Agriculture. 5 February 2015. Retrieved 1 March 2018.
- FAO FISHERIES TECHNICAL PAPER 441, Tevita Bainiloga Jnr, School of Chemistry, University College, University of New South Wales and Australian Defence Force Academy Canberra Australia
- Remminghorst and Rehm (2009). "Microbial Production of Alginate: Biosynthesis and Applications". Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press. ISBN 978-1-904455-36-3.
- FAO Fisheries Technical Paper, 2003
- Aizpurua-Olaizola, Oier; Navarro, Patricia; Vallejo, Asier; Olivares, Maitane; Etxebarria, Nestor; Usobiaga, Aresatz (2016-01-01). "Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes". Food Chemistry. 190: 614–621. doi:10.1016/j.foodchem.2015.05.117. PMID 26213018.
- Lansdown AB (2002). "Calcium: a potential central regulator in wound healing in the skin". Wound Repair Regen. 10 (5): 271–85. doi:10.1046/j.1524-475x.2002.10502.x. PMID 12406163.
- Stubbe, Birgit; Mignon, Arn; Declercq, Heidi; Vlierberghe, Sandra Van; Dubruel, Peter (2019). "Development of Gelatin-Alginate Hydrogels for Burn Wound Treatment". Macromolecular Bioscience. 19 (8): 1900123. doi:10.1002/mabi.201900123. ISSN 1616-5195. PMID 31237746.
- Büyüköz, M.; Erdal, E.; Altinkaya, S.A. (2016). "Nanofibrous gelatin scaffolds integrated with NGF-loaded alginate microspheres for brain tissue engineering". J. Tissue Eng. Regen. Med. 12 (2): e707–e719. doi:10.1002/term.2353. PMID 27863118. S2CID 206528926.
- Venkatesan, J; Bhatnagar, I; Manivasagan, P; Kang, K. H.; Kim, S. K. (2015). "Alginate composites for bone tissue engineering: A review". International Journal of Biological Macromolecules. 72: 269–81. doi:10.1016/j.ijbiomac.2014.07.008. PMID 25020082.
External links
- Alginate seaweed sources
- Alginate properties
- Alginate medical uses
- article Wired on Easy Cheese, describing sodium alginate