Isotopes of boron
Boron (5B) naturally occurs as isotopes 10B and 11B, the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-lives, the longest being that of 8B, with a half-life of only 770 milliseconds (ms) and 12B with a half-life of 20.2 ms. All other isotopes have half-lives shorter than 17.35 ms. Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for 7B and 9B) while those with mass above 11 mostly become carbon.
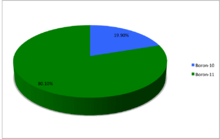
A chart showing the abundances of the naturally occurring isotopes of boron.
10B content may be as low as 19.1% and as high as 20.3% in natural samples. 11B is the remainder in such cases.[2] | ||||||||||||||||||||||||
| Standard atomic weight Ar, standard(B) |
| |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
List of isotopes
| Nuclide[4] [n 1] |
Z | N | Isotopic mass (Da)[5] [n 2][n 3] |
Half-life [resonance width] |
Decay mode [n 4] |
Daughter isotope [n 5] |
Spin and parity [n 6][n 7] |
Natural abundance (mole fraction) | |
|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | Normal proportion | Range of variation | |||||||
| 7B | 5 | 2 | 7.029712(27) | 570(14) × 10−24 s [801(20) keV] |
p | 6 Be [n 8] |
(3/2−) | ||
| 8B[n 9] | 5 | 3 | 8.0246073(11) | 770(3) ms | β+, α | 2 4 He |
2+ | ||
| 9B | 5 | 4 | 9.0133296(10) | 800(300) × 10−21 s [0.54(21) keV] |
p, α | 2 4 He |
3/2− | ||
| 10B | 5 | 5 | 10.012936862(16) | Stable | 3+ | 0.199(7) | 18.929–20.386 | ||
| 11B | 5 | 6 | 11.009305167(13) | Stable | 3/2− | 0.801(7) | 79.614–81.071 | ||
| 12B | 5 | 7 | 12.0143526(14) | 20.20(2) ms | β− (98.4%) | 12 C |
1+ | ||
| β−, α (1.6%) | 8 Be [n 10] | ||||||||
| 13B | 5 | 8 | 13.0177800(11) | 17.33(17) ms | β− (99.72%) | 13 C |
3/2− | ||
| β−, n (0.28%) | 12 C | ||||||||
| 14B | 5 | 9 | 14.025404(23) | 12.5(5) ms | β− (93.96%) | 14 C |
2− | ||
| β−, n (6.04%) | 13 C | ||||||||
| 15B | 5 | 10 | 15.031088(23) | 9.93(7) ms | β−, n (93.6%) | 14 C |
3/2− | ||
| β− (6.0%) | 15 C | ||||||||
| β−, 2n (0.4%) | 13 C | ||||||||
| 16B | 5 | 11 | 16.039842(26) | > 4.6 × 10−21 s |
n | 15 B |
0− | ||
| 17B[n 11] | 5 | 12 | 17.04693(22) | 5.08(5) ms | β−, n (63.0%) | 16 C |
(3/2−) | ||
| β− (22.1%) | 17 C | ||||||||
| β−, 2n (11.0%) | 15 C | ||||||||
| β−, 3n (3.5%) | 14 C | ||||||||
| β−, 4n (0.4%) | 13 C | ||||||||
| 18B | 5 | 13 | 18.05560(22) | < 26 ns | n | 17 B |
(2−) | ||
| 19B[n 11] | 5 | 14 | 19.06417(56) | 2.92(13) ms | β−, n (71%) | 18 C |
3/2−# | ||
| β−, 2n (17%) | 17 C | ||||||||
| β− (12%) | 19 C | ||||||||
| 20B[6] | 5 | 15 | 20.07348(86)# | [2.50(9) MeV] | n | 19 B |
(1−, 2−) | ||
| 21B[6] | 5 | 16 | 21.08302(97)# | < 260 ns [2.47(19) MeV] |
2n | 19 B |
(3/2−)# | ||
- mB – Excited nuclear isomer.
- ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
-
Modes of decay:
n: Neutron emission p: Proton emission - Bold symbol as daughter – Daughter product is stable.
- ( ) spin value – Indicates spin with weak assignment arguments.
- # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
- Subsequently decays by double proton emission to 4He for a net reaction of 7B → 4He + 3 1H
- Has 1 halo proton
- Immediately decays into two α particles, for a net reaction of 12B → 3 4He + e−
- Has 2 halo neutrons
- Neutrinos from boron-8 beta decays within the sun are an important background to dark matter direct detection experiments.[7] They are the first component of the neutrino floor that dark matter direct detection experiments are expected to eventually encounter.
Applications
Boron-10
Boron-10 is used in boron neutron capture therapy (BNCT) as an experimental treatment of some brain cancers.
References
- "Atomic Weights and Isotopic Compositions for All Elements". National Institute of Standards and Technology. Retrieved 2008-09-21.
- Szegedi, S.; Váradi, M.; Buczkó, Cs. M.; Várnagy, M.; Sztaricskai, T. (1990). "Determination of boron in glass by neutron transmission method". Journal of Radioanalytical and Nuclear Chemistry Letters. 146 (3): 177. doi:10.1007/BF02165219.
- Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- Half-life, decay mode, nuclear spin, and isotopic composition is sourced in:
Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001. - Wang, M.; Audi, G.; Kondev, F. G.; Huang, W. J.; Naimi, S.; Xu, X. (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/3/030003.
- Leblond, S.; et al. (2018). "First observation of 20B and 21B". Physical Review Letters. 121 (26): 262502–1–262502–6. arXiv:1901.00455. doi:10.1103/PhysRevLett.121.262502. PMID 30636115.
- Cerdeno, David G.; Fairbairn, Malcolm; Jubb, Thomas; Machado, Pedro; Vincent, Aaron C.; Boehm, Celine (2016). "Physics from solar neutrinos in dark matter direct detection experiments". JHEP. 2016 (5): 118. arXiv:1604.01025. Bibcode:2016JHEP...05..118C. doi:10.1007/JHEP05(2016)118.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.