Group 3 element
Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or bifurcated to include both La-Ac and Lu-Lr. When the group is understood to contain all of the lanthanides, it subsumes the rare-earth metals. Yttrium, and less frequently scandium, are sometimes also counted as rare-earth metals.
| Group 3 in the periodic table | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| ↓ Period | |||||||||
| 4 |  21 Transition metal | ||||||||
| 5 |  39 Transition metal | ||||||||
| 6 |  57 Lanthanide | ||||||||
| 6 |  71 Lanthanide | ||||||||
| 7 |  89 Actinide | ||||||||
| 7 | Lawrencium (Lr*) 103 Actinide | ||||||||
|
* Whether the elements lanthanum (La) and actinium (Ac) or the elements lutetium (Lu) and lawrencium (Lr) are in group 3 is disputed. See composition of group 3. | |||||||||
|
Legend
| |||||||||
Three group 3 elements occur naturally: scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. While the choice of lutetium would be in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors, the choice of lanthanum is in accordance with the trends in the s-block, which the group 3 elements are chemically somewhat similar to (as are the lanthanides and actinides in general, as well as the heavy elements of groups 4 and 5). They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, oganesson.
History
In 1787, Swedish part-time chemist Carl Axel Arrhenius found a heavy black rock near the Swedish village of Ytterby, Sweden (part of the Stockholm Archipelago).[1] Thinking that it was an unknown mineral containing the newly discovered element tungsten,[2] he named it ytterbite.[n 1] Finnish scientist Johan Gadolin identified a new oxide or "earth" in Arrhenius' sample in 1789, and published his completed analysis in 1794;[3] in 1797, the new oxide was named yttria.[4] In the decades after French scientist Antoine Lavoisier developed the first modern definition of chemical elements, it was believed that earths could be reduced to their elements, meaning that the discovery of a new earth was equivalent to the discovery of the element within, which in this case would have been yttrium.[n 2] Until the early 1920s, the chemical symbol "Yt" was used for the element, after which "Y" came into common use.[5] Yttrium metal was first isolated in 1828 when Friedrich Wöhler heated anhydrous yttrium(III) chloride with potassium to form metallic yttrium and potassium chloride.[6][7]
In 1869, Russian chemist Dmitri Mendeleev published his periodic table, which had empty spaces for elements directly above and under yttrium.[8] Mendeleev made several predictions on the upper neighbor of yttrium, which he called eka-boron. Swedish chemist Lars Fredrik Nilson and his team discovered the missing element in the minerals euxenite and gadolinite and prepared 2 grams of scandium(III) oxide of high purity.[9][10] He named it scandium, from the Latin Scandia meaning "Scandinavia". Chemical experiments on the element proved that Mendeleev's suggestions were correct; along with discovery and characterization of gallium and germanium this proved the correctness of the whole periodic table and periodic law. Nilson was apparently unaware of Mendeleev's prediction, but Per Teodor Cleve recognized the correspondence and notified Mendeleev.[11] Metallic scandium was produced for the first time in 1937 by electrolysis of a eutectic mixture, at 700–800 °C, of potassium, lithium, and scandium chlorides.[12]
In 1751, the Swedish mineralogist Axel Fredrik Cronstedt discovered a heavy mineral from the mine at Bastnäs, later named cerite. Thirty years later, the fifteen-year-old Vilhelm Hisinger, from the family owning the mine, sent a sample of it to Carl Scheele, who did not find any new elements within. In 1803, after Hisinger had become an ironmaster, he returned to the mineral with Jöns Jacob Berzelius and isolated a new oxide which they named ceria after the dwarf planet Ceres, which had been discovered two years earlier.[13] Ceria was simultaneously independently isolated in Germany by Martin Heinrich Klaproth.[14] Between 1839 and 1843, ceria was shown to be a mixture of oxides by the Swedish surgeon and chemist Carl Gustaf Mosander, who lived in the same house as Berzelius: he separated out two other oxides which he named lanthana and didymia.[15] He partially decomposed a sample of cerium nitrate by roasting it in air and then treating the resulting oxide with dilute nitric acid.[16] Since lanthanum's properties differed only slightly from those of cerium, and occurred along with it in its salts, he named it from the Ancient Greek λανθάνειν [lanthanein] (lit. to lie hidden).[14] Relatively pure lanthanum metal was first isolated in 1923.[17]
Lutetium was independently discovered in 1907 by French scientist Georges Urbain,[18] Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James[19] as an impurity in the mineral ytterbia, which was thought by most chemists to consist entirely of ytterbium. Welsbach proposed the names cassiopeium for element 71 (after the constellation Cassiopeia) and aldebaranium (after the star Aldebaran) for the new name of ytterbium but these naming proposals were rejected, although many German scientists in the 1950s called the element 71 cassiopeium. Urbain chose the names neoytterbium (Latin for "new ytterbium") for ytterbium and lutecium (from Latin Lutetia, for Paris) for the new element. The dispute on the priority of the discovery is documented in two articles in which Urbain and von Welsbach accuse each other of publishing results influenced by the published research of the other.[20][21] The Commission on Atomic Mass, which was responsible for the attribution of the names for the new elements, settled the dispute in 1909 by granting priority to Urbain and adopting his names as official ones. An obvious problem with this decision was that Urbain was one of the four members of the commission.[22] The separation of lutetium from ytterbium was first described by Urbain and the naming honor therefore went to him, but neoytterbium was eventually reverted to ytterbium and in 1949, the spelling of element 71 was changed to lutetium.[23][24] Ironically, Charles James, who had modestly stayed out of the argument as to priority, worked on a much larger scale than the others, and undoubtedly possessed the largest supply of lutetium at the time.[25]
André-Louis Debierne, a French chemist, announced the discovery of actinium in 1899. He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium. In 1899, Debierne described the substance as similar to titanium[26] and (in 1900) as similar to thorium.[27] Friedrich Oskar Giesel independently discovered actinium in 1902[28] as a substance being similar to lanthanum and called it "emanium" in 1904.[29] After a comparison of the substances half-lives determined by Debierne,[30] Hariett Brooks in 1904, and Otto Hahn and Otto Sackur in 1905, Debierne's chosen name for the new element was retained because it had seniority, despite the contradicting chemical properties he claimed for the element at different times.[31][32]

Lawrencium was first synthesized by Albert Ghiorso and his team on February 14, 1961, at the Lawrence Radiation Laboratory (now called the Lawrence Berkeley National Laboratory) at the University of California in Berkeley, California, United States. The first atoms of lawrencium were produced by bombarding a three-milligram target consisting of three isotopes of the element californium with boron-10 and boron-11 nuclei from the Heavy Ion Linear Accelerator (HILAC).[33] The nuclide 257103 was originally reported, but then this was reassigned to 258103. The team at the University of California suggested the name lawrencium (after Ernest O. Lawrence, the inventor of cyclotron particle accelerator) and the symbol "Lw",[33] for the new element, but "Lw" was not adopted, and "Lr" was officially accepted instead. Nuclear-physics researchers in Dubna, Soviet Union (now Russia), reported in 1967 that they were not able to confirm American scientists' data on 257103.[34] Two years earlier, the Dubna team reported 256103.[35] In 1992, the IUPAC Trans-fermium Working Group officially recognized element 103, confirmed its naming as lawrencium, with symbol "Lr", and named the nuclear physics teams at Dubna and Berkeley as the co-discoverers of lawrencium.[36]
If lutetium and lawrencium are considered to be group 3 elements, then extrapolation from the Aufbau principle would predict that the next element in the group should be element 153, unpenttrium (Upt). According to the principle, unpenttrium should have an electronic configuration of [Og]8s25g186f147d1[n 3] and filling the 5g-subshell should be stopped at element 138. However, elements beyond 120 are predicted to stop following the Aufbau principle: the 7d-orbitals are calculated to start being filled on element 137, while the 5g-subshell closes only at element 144, after filling of 7d-subshell begins. Therefore, it is hard to calculate which element should be the next group 3 element.[37] Calculations suggest that unpentpentium (Upp, element 155) could also be the next group 3 element,[38] as could unpentseptium (Ups, element 157).[39] If lanthanum and actinium are considered group 3 elements, then element 121, unbiunium (Ubu), should be the fifth group 3 element. The element is calculated to have electronic configuration of [Og]8s28p1/21, with an anomalous p-electron similar to that of lawrencium.[37] The synthesis of unbiunium was attempted unsuccessfully in 1977,[40] though its proximity to known elements and advances in accelerator technology may enable its creation in the near future.[41][42] No other synthesis experiments have been conducted.
Characteristics
Chemical
| Z | Element | Electron configuration |
|---|---|---|
| 21 | scandium | 2, 8, 9, 2 |
| 39 | yttrium | 2, 8, 18, 9, 2 |
| 57 | lanthanum | 2, 8, 18, 18, 9, 2 |
| 71 | lutetium | 2, 8, 18, 32, 9, 2 |
| 89 | actinium | 2, 8, 18, 32, 18, 9, 2 |
| 103 | lawrencium | 2, 8, 18, 32, 32, 8, 3 |
Like other groups, the members of this family show patterns in their electron configurations, especially the outermost shells, resulting in trends in chemical behavior. However, lawrencium is an exception, since its last electron is transferred to the 7p1/2 subshell due to relativistic effects.[43][44]
Most of the chemistry has been observed only for the first three members of the group; chemical properties of both actinium and especially lawrencium are not well-characterized. The remaining elements of the group (scandium, yttrium, lutetium) are reactive metals with high melting points (1541 °C, 1526 °C, 1652 °C respectively). They are usually oxidized to the +3 oxidation state, even though scandium,[45] yttrium[46][47] and lanthanum[17] can form lower oxidation states. The reactivity of the elements, especially yttrium, is not always obvious due to the formation of a stable oxide layer, which prevents further reactions. Scandium(III) oxide, yttrium(III) oxide, lanthanum(III) oxide and lutetium(III) oxide are white high-temperature-melting solids. Yttrium(III) oxide and lutetium(III) oxide exhibit weak basic character, but scandium(III) oxide is amphoteric.[48] Lanthanum(III) oxide is strongly basic.
Physical
Elements that show tripositive ions with electronic configuration of a noble gas (scandium, yttrium, lanthanum, actinium) show a clear trend in their physical properties, such as hardness. At the same time, if group 3 is continued with lutetium and lawrencium, several trends are broken. For example, scandium and yttrium are both soft metals. Lanthanum is soft as well; all these elements have their outermost electrons quite far from the nucleus compared to the nuclei charges. Due to the lanthanide contraction, lutetium, the last in the lanthanide series, has a significantly smaller atomic radius and a higher nucleus charge,[49] thus making the extraction of the electrons from the atom to form metallic bonding more difficult, and thus making the metal harder. However, lutetium suits the previous elements better in several other properties, such as melting[50] and boiling points.[51] Very little is known about lawrencium, and none of its physical properties have been confirmed.[52][53]
| Name | Scandium | Yttrium | Lanthanum | Lutetium | Actinium | Lawrencium |
|---|---|---|---|---|---|---|
| Melting point[50] | 1814 K, 1541 °C | 1799 K, 1526 °C | 1193 K, 920 °C | 1925 K, 1652 °C | 1323 K, 1050 °C | ? 1900 K, ? 1627 °C |
| Boiling point[51] | 3109 K, 2836 °C | 3609 K, 3336 °C | 3737 K, 3464 °C | 3675 K, 3402 °C | 3471 K, 3198 °C | ? |
| Density | 2.99 g·cm−3[55] | 4.47 g·cm−3[56] | 6.162 g·cm−3 | 9.84 g·cm−3 | 10 g·cm−3 | ? 16 g·cm−3 |
| Appearance | silver metallic | silver white | gray | silver gray | silvery | ? |
| Atomic radius[49] | 162 pm | 180 pm | 187 pm | 174 pm | 215 pm | ? |
Composition of group 3
Although scandium and yttrium are always the first two elements in group 3, the identity of the next two elements is not completely settled. They are commonly lanthanum and actinium, and less often lutetium and lawrencium. The two variants originate from historical difficulties in placing the lanthanides in the periodic table, and arguments as to where the f block elements start and end.[57]
The detachment of the lanthanides from the main body of the periodic table has been attributed to the Czech chemist Bohuslav Brauner who, in 1902, allocated all of them ("Ce etc.") to one position in group 4, below zirconium. This arrangement was referred to as the "asteroid hypothesis", in analogy to asteroids occupying a single orbit in the solar system. Before this time the lanthanides were generally (and unsuccessfully) placed throughout groups I to VIII of the older 8-column form of periodic table. Although predecessors of Brauner's 1902 arrangement are recorded from as early as 1895, he is known to have referred to the "chemistry of asteroids" in an 1881 letter to Mendeleev. Other authors assigned all of the lanthanides to either group 3, groups 3 and 4, or groups 2, 3 and 4. In 1922 Niels Bohr continued the detachment process by locating the lanthanides between the s- and d-blocks. In 1949 Glenn T. Seaborg (re)introduced the form of periodic table that is popular today, in which the lanthanides and actinides appear as footnotes. Seaborg first published his table in a classified report dated 1944. It was published again by him in 1945 in Chemical and Engineering News, and in the years up to 1949 several authors commented on, and generally agreed with, Seaborg's proposal. In that year he noted that the best method for presenting the actinides seemed to be by positioning them below, and as analogues of, the lanthanides.[58]
It has been claimed that such arguments are proof that, "it is a mistake to break the [periodic] system into sharply delimited blocks".[59] A third common variant shows the two positions below yttrium as being occupied by the lanthanides and the actinides. A fourth variant shows group 3 bifurcating after Sc-Y, into an La-Ac branch, and an Lu-Lr branch.[60]
In 1895, even before the discovery of lutetium, Hans Peter Jørgen Julius Thomsen considered lanthanum to ytterbium to form a 14-element series.[61] Since 1921, many chemical and physical arguments have been made in support of lutetium and lawrencium[62][63] but the majority of authors seem either unconvinced by them or unaware of them.[64][65] Most working chemists are not aware there is any controversy.[65] In December 2015 an IUPAC project was established to make a recommendation on the matter, considering only the first two alternatives as possibilities.[66]
Lanthanum and actinium
 La and Ac below Y |
Lanthanum and actinium are commonly depicted as the remaining group 3 members.[67][n 4] It has been suggested that this layout originated in the 1940s, with the appearance of periodic tables relying on the ground-state electron configurations of the elements and the notion of the differentiating electron. The ground-state configurations of caesium, barium and lanthanum are [Xe]6s1, [Xe]6s2 and [Xe]5d16s2. Lanthanum thus emerges with a 5d differentiating electron and on these grounds it was considered to be "in group 3 as the first member of the d-block for period 6".[68] A superficially consistent set of electron configurations is then seen in group 3: scandium [Ar]3d14s2, yttrium [Kr]4d15s2 and lanthanum [Xe]5d16s2. Still in period 6, ytterbium was assigned an electron configuration of [Xe]4f135d16s2 and lutetium [Xe]4f145d16s2, "resulting in a 4f differentiating electron for lutetium and firmly establishing it as the last member of the f-block for period 6".[68] Later spectroscopic work found that the electron configuration of ytterbium was in fact [Xe]4f146s2. This meant that ytterbium and lutetium—the latter with [Xe]4f145d16s2—both had 14 f-electrons, "resulting in a d- rather than an f- differentiating electron" for lutetium and making it an "equally valid candidate" with [Xe]5d16s2 lanthanum, for the group 3 periodic table position below yttrium.[68] Lanthanum has the advantage of incumbency since the 5d1 electron appears for the first time in its structure whereas it appears for the third time in lutetium, having also made a brief second appearance in gadolinium[69] (though similar logic would also lead to thorium getting the 6d2 position, having incumbency over rutherfordium).
Ground-state gas-phase configurations consider only isolated atoms as opposed to bonding atoms in compounds (the latter being more relevant for chemistry), which often show different configurations.[70] Moreover, the lowest levels of two different configurations often are separated by only very small energies, that are minuscule compared to the spreading of J-levels of each configuration (e.g. terbium, where the 285 cm−1 difference between [Xe]4f85d16s2 and the ground state [Xe]4f95s2 is much less than 1% of this spreading), making which configuration happens to be the ground state chemically quite irrelevant.[61] It is the dominant electron configuration of atoms in chemical environments, and not free gaseous atoms in a vacuum, that can rationalise qualitative chemical behaviour.[71]
The form with lanthanum under yttrium also creates an inconsistency in the treatment of thorium, which has no f-electrons in the ground-state (being [Rn]6d27s2), similar to actinium as [Rn]6d17s2; yet it places thorium in the f-block but not actinium.[72] Considering only ground-state gas-phase configurations, thorium [Rn]6d27s2 by itself is just as good a homologue to zirconium [Kr]4d25s2 as lanthanum [Xe]5d16s2 is to scandium [Ar]3d14s2;[61] yet thorium is invariably placed in the f-block. Thorium thus demonstrates that the possession of an f electron in the ground-state gas-phase configuration of an element is not necessary for it to belong to the f-block.[73] Additionally, this form necessitates a split d-block if expanded to a 32-column periodic table.[73]
In terms of chemical behaviour,[74] and trends going down group 3 (if Sc-Y-La is chosen) for properties such as melting point, electronegativity and ionic radius,[75][76] scandium, yttrium, lanthanum and actinium are similar to their group 1–2 counterparts, but at variance with the other groups in the early d-block. In this variant, the number of f electrons in the most common (trivalent) ions of the f-block elements consistently matches their position in the f-block.[77] For example, the f-electron counts for the trivalent ions of the first three f-block elements are Ce 1, Pr 2 and Nd 3.[78] However, outside the lanthanides there does not exist a typical oxidation state across any period of a block,[79] and the reason for this singular behaviour of the lanthanides in fact has very little to do with the electron configurations of the elements concerned,[80] which on the face of it would seem to predict a preferred +2 oxidation state as they are mostly [Xe]4fn6s2 (lanthanides) or [Rn]5fn7s2 (actinides).[81]
It is important to realize that the electronic structures listed ... are those of the neutral (unionized) gaseous atoms, whereas it is the electronic structure of the ions and compounds that we are chiefly concerned with in chemistry. The relationship of the electronic structure of the gaseous atom of an element to that of its compounds can be rather complicated. For example, in the case of the actinide and lanthanide elements, one would not necessarily predict the predominance of the III oxidation state from the electronic structures of the gaseous atoms; there are usually only two so-called "valence electrons," the 7s or 6s electrons, which might indicate a preference for the II oxidation state.
Apparently, specific factors in the crystal structure of, and the aquation (hydration) energies of, the compounds and ions are important in determining the stability of the III oxidation state. Thus, the characteristic tripositive oxidation state of the lanthanide elements is not related directly to the number of "valence electrons" outside the 4f subshell, but is the somewhat accidental result of a nearly constant small difference between large energy terms (ionization potentials on the one hand, and hydration and crystal energies on the other) which persists over an interval of fourteen atomic numbers. Therefore, if we could somehow have a very extended Periodic Table of Elements containing numerous "f" transition series, we might expect that the 5f, rather than the 4f, elements would be regarded as more nearly representative of such f series.[81]— Glenn T. Seaborg, Origin of the Actinide Concept (1991)
Similarity of chemistry is, in addition, not the only factor that needs to be considered for periodic table placement. Tungsten and uranium chemically resemble each other (and were placed in the same group before Seaborg's clarification of the actinides), in a manner that is not worse than the resemblances between tin and lead, or between antimony and bismuth, both of which are universally considered to belong in the same group.[61] Moreover, the resemblance between aluminium and scandium, which are placed in different groups, is actually stronger in some ways than that between aluminium and gallium, which are in the same group.[82] The same is true of the relationship of beryllium and magnesium to zinc, which is in some ways stronger than their relationship to calcium.[83]
Lutetium and lawrencium
 Lu and Lr below Y |
In other tables, lutetium and lawrencium are the remaining group 3 members.[n 5] Early techniques for chemically separating scandium, yttrium and lutetium relied on the fact that these elements occurred together in the so-called "yttrium group" whereas La and Ac occurred together in the "cerium group".[68] Accordingly, lutetium rather than lanthanum was assigned to group 3 by some chemists in the 1920s and 30s. The phenomenon of different separation groups is caused by increasing basicity with increasing radius, and does not constitute a fundamental reason to show Lu, rather than La, below Y. Thus, among the Group 2 alkaline earth metals, Mg (less basic) belongs in the "soluble group" and Ca, Sr and Ba (more basic) occur in the "ammonium carbonate group". Nevertheless, Mg, Ca, Sr and Ba are routinely collocated in Group 2 of the periodic table.[84]
Several physicists in the 1950s and '60s favoured lutetium, in light of a comparison of several of its physical properties with those of lanthanum.[68] Among the prominent adherents of this form have been Lev Landau and Evgeny Lifshitz, who wrote in their Course of Theoretical Physics (1958):[85]
In books of chemistry, lutetium is usually placed in the rare-earth elements. This, however, is incorrect, since the 4f shell is complete in lutetium; it must therefore be placed in the platinum group [which they considered to be La+Lu–Pt]...
— Landau and Lifshitz, Course of Theoretical Physics, Vol. 3: Quantum Mechanics: Non-Relativistic Theory (1958)
This arrangement, in which lanthanum is the first member of the f-block, is disputed by some authors since lanthanum lacks any f-electrons. It has been argued that this is not a valid concern given other periodic table anomalies, such as thorium.[86] The binding energies of the 4f levels of excited states of lanthanum that contain a 4f electron clearly show that lanthanum's 4f orbitals are not hydrogenic. In other words, in hydrogen through barium, the 4f orbitals are far enough from the nucleus that when analysing them, one can approximate the core and remaining electrons as a point charge; starting from lanthanum, this ceases to be the case, with lanthanum showing 4f levels more similar to those of the following rare earths.[87] These low-lying empty f orbitals, which lutetium lacks,[72] contribute measurably to the bonding in some lanthanum compounds, for example in lanthanum(III) fluoride (LaF3). While this contribution is small, it is greater for lanthanum than for any other lanthanide, considering for each the analogous LnF3 compound; meanwhile, the Lu–F 4f–2p bond order in LuF3 is less than the analogous one of IrF3, with iridium well into the 5d block.[88] And while the trivalent lanthanides Pr3+ through Yb3+ show characteristic narrow bands with their positions almost completely independent on the ligands, the following 5d elements (along with the 3d and 4d elements) behave significantly differently; while both types of elements show electron-transfer bands, ligand field theory becomes important for these d elements.[80] The order of involvement of 4f in lanthanum is similar to that of 5f in thorium; that of 4f in cerium is similar to that of 5f in uranium.[61]
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
| hcp | hcp | bcc | bcc | ~bcc | bcc | hcp | fcc | fcc | ~hcp |
| Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd |
| hcp | hcp | bcc | bcc | hcp | hcp | fcc | fcc | fcc | ~hcp |
| Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg |
| hcp | hcp | bcc | bcc | hcp | hcp | fcc | fcc | fcc | ~hcp |
| Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn |
| hcp | hcp | bcc | bcc | hcp | hcp | fcc | bcc | bcc | bcc |
Lanthanum has the dhcp crystal structure as the most stable one at standard conditions, and actinium is fcc; whereas scandium, yttrium, lutetium, and lawrencium (the last predicted) show the hcp crystal structure. The dhcp crystal structure is only known for some rare earths and actinides in the f-block and is unknown elsewhere on the periodic table.[87] This constitutes an anomaly in the otherwise completely regular variation of the crystal structures of the nonmagnetic transition metals with their valencies (except for the late 6d metals, which should be anomalous due to strong relativistic effects for those superheavy elements),[89][90] and is a sign of 4f band involvement for lanthanum, because lanthanum without 4f involvement would be expected to be hcp like scandium, yttrium, and lutetium. Instead, the pressure-temperature phase diagram of lanthanum is isomorphic to those of the uncontroversial 4f metals praseodymium and neodymium.[89]
Karl Gschneidner, analysing the melting points of the lanthanides in a 1971 article, reached the conclusion that it was likely that 4f, 5d, 6s, and 6p electrons were all involved in the bonding of lanthanide metals except for lutetium, where 4f electrons were not found to be involved.[91] The fact that lanthanum was demonstrated to be a 4f-band metal (with about 0.17 electrons per atom in fcc lanthanum, which is metastable at standard conditions)[92] whereas the 4f shell appears to have no influence on the metallic properties of lutetium, has been used as an argument to place lutetium in group 3 instead of lanthanum.[89] The 4f occupancy in solid lanthanum may explain some of its properties, such as its low melting point (La 920 °C, versus Sc 1541 °C, Y 1526 °C, Lu 1652 °C)[91] low Debye temperature, and anomalously high superconducting transition temperature at all pressures.[89] Indeed, if lanthanum is treated as a d-block element, it constitutes anomalies in the trends of superconducting transition temperatures at a variety of pressures, all of which are removed if lutetium is put in its place.[87][89] Jörg Wittig, considering this problem in 1973, found it likely that this small 4f band involvement in lanthanum "represents the screening charge of a 4f scattering resonance safeguarded deep in the interior of the lanthanum ion core", similarly to cerium: this is in agreement with Gschneidner's model. The difficulty in observing this would then be due to the strong d resonance that this 4f virtual bound state also has. This is confirmed by the alloy LaAl2, whose 16% lower Debye temperature and higher electronic specific heat coefficients compared to LuAl2 reflect "directly the additional 4f density of states at the Fermi surface".[89]
Scandium, yttrium, and lutetium show a more consistent set of electron configurations matching the global trend on the periodic table: the 5d metals then all add a closed 4f14 shell. For example, the shift from yttrium [Kr]4d15s2 to lutetium [Xe]4f145d16s2 exactly parallels that from zirconium [Kr]4d25s2 to hafnium [Xe]4f145d26s2.[68] The inclusion of lutetium rather than lanthanum also homogenises the 5d transition series: trends in atomic size, coordination number, and relative abundance of metal–oxygen bonds all reveal that lutetium is closer than lanthanum to the behaviour of the uncontroversial 5d metals hafnium through mercury.[93] The same is true considering conduction band structures of the elements: lutetium has a transition-metal-like conduction band structure, but lanthanum does not.[94]
As for lawrencium, its gas phase ground-state atomic electron configuration was confirmed in 2015 as [Rn]5f147s27p1. Such a configuration represents another periodic table anomaly, regardless of whether lawrencium is located in the f-block or the d-block, as the only potentially applicable p-block position has been reserved for nihonium with its predicted configuration of [Rn]5f146d107s27p1.[95] However, it is expected that in the condensed phase and in chemical environments lawrencium has the expected 6d occupancy, and simple modelling studies suggest it will behave like a lanthanide,[96] in particular being a homologue of lutetium. Lawrencium's return to +3 as the only stable oxidation state and being predicted to form a trivalent metal is distinct from the behaviour of the other late actinides fermium, mendelevium, and nobelium, which have a tendency towards forming lower oxidation states and form (or are predicted to form) divalent metals; it also makes an exception to the actinide contraction generally being larger than the analogous lanthanide contraction at the end of both series.[97] Meanwhile, actinium has a band structure with itinerant 5f electrons, that is similar to those of lanthanum and praseodymium;[98] the 5f bands are in the same region as and hybridise strongly with the 6d and 7s bands, with the width of the 5f band increasing with pressure.[99]
While scandium, yttrium and lutetium (and lawrencium, so far as its chemistry is known) do often behave like trivalent versions of the group 1–2 metals, being hard class-A cations mostly restricted to the group oxidation state, they are not the only elements in the d-block or f-block that do so. The early transition metals zirconium and hafnium in group 4, as well as niobium and tantalum in group 5, also display such behaviour, as does the actinide thorium. (The heavy group 4 elements and thorium are tetravalent; the heavy group 5 elements are pentavalent.)[100][101] The physical properties of the group 3 elements are affected by the presence of a d electron, which forms more localised bonds within the metals than the p electrons in the similar group 13 metals;[102] exactly the same situation is found comparing group 4 to group 14.[103] Trends going down group 3 (if Sc-Y-Lu is chosen) for properties such as melting point, electronegativity and ionic radius, are similar to those found among their group 4–8 counterparts in the same block, as noted by William B. Jensen in an often-cited 1982 article in which he argued for this placement.[68] In this variant, the number of f electrons in the gaseous forms of the f-block atoms usually matches their position in the f-block. For example, the f-electron counts for the first five f-block elements are La 0, Ce 1, Pr 3, Nd 4 and Pm 5.[68]
Lanthanides and actinides
 Markers below Y |
A few authors position all thirty lanthanides and actinides in the two positions below yttrium (usually via footnote markers). This variant, which is stated in the 2005 Red Book to be the IUPAC-agreed version as of 2005 (a number of later versions exist, and the last update is from 1 December 2018),[104][n 6] emphasizes similarities in the chemistry of the 15 lanthanide elements (La–Lu), possibly at the expense of ambiguity as to which elements occupy the two group 3 positions below yttrium, and a 15-column wide f block (there can only be 14 elements in any row of the f block).[n 7] However, this similarity does not extend to the 15 actinide elements (Ac–Lr), which show a much wider variety in their chemistries.[61] This form moreover reduces the f-block to a degenerate branch of group 3 of the d-block; it dates back to the 1920s when the lanthanides were thought to have their f electrons as core electrons, which is now known to be false. It is also false for the actinides, many of which show stable oxidation states above +3.[106]
La-Ac and Lu-Lr
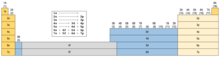
In this variant, group 3 bifurcates after Sc-Y into a La-Ac branch, and a Lu-Lr branch. This arrangement is consistent with the hypothesis that arguments in favour of either Sc-Y-La-Ac or Sc-Y-Lu-Lr based on chemical and physical data are inconclusive.[107] As noted, trends going down Sc-Y-La-Ac match trends in groups 1−2[108] whereas trends going down Sc-Y-Lu-Lr better match trends in groups 4−10.[68]
The bifurcation of group 3 is a throwback to the Mendeleev eight column-form in which seven of the main groups each have two subgroups. Tables featuring a bifurcated group 3 have been periodically proposed since that time.[n 8]
Occurrence
Scandium, yttrium, lanthanum, and lutetium tend to occur together with the other lanthanides (except promethium) in the Earth's crust, and are often harder to extract from their ores. The abundance of elements in Earth's crust for group 3 is quite low—all the elements in the group are uncommon, the most abundant being yttrium with abundance of approximately 30 parts per million (ppm); the abundance of scandium is 16 ppm, while that of lutetium is about 0.5 ppm. The abundance of lanthanum is greater, being about 35 ppm. For comparison, the abundance of copper is 50 ppm, that of chromium is 160 ppm, and that of molybdenum is 1.5 ppm.[109]
Scandium is distributed sparsely and occurs in trace amounts in many minerals.[110] Rare minerals from Scandinavia[111] and Madagascar[112] such as gadolinite, euxenite, and thortveitite are the only known concentrated sources of this element, the latter containing up to 45% of scandium in the form of scandium(III) oxide.[111] Yttrium has the same trend in occurrence places; it is found in lunar rock samples collected during the American Apollo Project in a relatively high content as well.[113]

The principal commercially viable ore of lutetium is the rare-earth phosphate mineral monazite, (Ce,La,etc.)PO4, which contains 0.003% of the element. The main mining areas are China, United States, Brazil, India, Sri Lanka and Australia. Pure lutetium metal is one of the rarest and most expensive of the rare-earth metals with the price about US$10,000/kg, or about one-fourth that of gold. Lanthanum is much more common, being the second most abundant rare earth, and in addition to monazite can also be extracted economically from bastnäsite.[114][115]
Production
The most available element in group 3 is yttrium, with annual production of 8,900 tonnes in 2010. Yttrium is mostly produced as oxide, by a single country, China (99%).[116] Lutetium and scandium are also mostly obtained as oxides, and their annual production by 2001 was about 10 and 2 tonnes, respectively.[117]
Group 3 elements are mined only as a byproduct from the extraction of other elements.[118] The metallic elements are extremely rare; the production of metallic yttrium is about a few tonnes, and that of scandium is in the order of 10 kg per year;[118][119] production of lutetium is not calculated, but it is certainly small. The elements, after purification from other rare-earth metals, are isolated as oxides; the oxides are converted to fluorides during reactions with hydrofluoric acid.[120] The resulting fluorides are reduced with alkaline earth metals or alloys of the metals; metallic calcium is used most frequently.[120] For example:
- Sc2O3 + 3 HF → 2 ScF3 + 3 H2O
- 2 ScF3 + 3 Ca → 3 CaF2 + 2 Sc
Biological chemistry
Group 3 elements are generally hard metals with low aqueous solubility, and have low availability to the biosphere. No group 3 element has any documented biological role in living organisms. The radioactivity of the actinides generally makes them highly toxic to living cells, causing radiation poisoning.
Scandium has no biological role, but it is found in living organisms. Once reached a human, scandium concentrates in the liver and is a threat to it; some its compounds are possibly carcinogenic, even through in general scandium is not toxic.[121] Scandium is known to have reached the food chain, but in trace amounts only; a typical human takes in less than 0.1 micrograms per day.[121] Once released into the environment, scandium gradually accumulates in soils, which leads to increased concentrations in soil particles, animals and humans. Scandium is mostly dangerous in the working environment, due to the fact that damps and gases can be inhaled with air. This can cause lung embolisms, especially during long-term exposure. The element is known to damage cell membranes of water animals, causing several negative influences on reproduction and on the functions of the nervous system.[121]
Yttrium has no known biological role, though it is found in most, if not all, organisms and tends to concentrate in the liver, kidney, spleen, lungs, and bones of humans.[122] There is normally as little as 0.5 milligrams found within the entire human body; human breast milk contains 4 ppm.[123] Yttrium can be found in edible plants in concentrations between 20 ppm and 100 ppm (fresh weight), with cabbage having the largest amount.[123] With up to 700 ppm, the seeds of woody plants have the highest known concentrations.[123]
Lutetium has no biological role as well, but it is found even in the highest known organism, the humans, concentrating in bones, and to a lesser extent in the liver and kidneys.[124] Lutetium salts are known to cause metabolism and they occur together with other lanthanide salts in nature; the element is the least abundant in the human body of all lanthanides.[124] Human diets have not been monitored for lutetium content, so it is not known how much the average human takes in, but estimations show the amount is only about several micrograms per year, all coming from tiny amounts taken by plants. Soluble lutetium salts are mildly toxic, but insoluble ones are not.[124] Lanthanum is not essential for humans and has a low to moderate level of toxicity. However, it is essential for the methanotrophic bacterium Methylacidiphilum fumariolicum SolV, although the general similarity of the rare earths means that it may be substituted by some of the other early lanthanides with no ill effects.[125]
The high radioactivity of lawrencium would make it highly toxic to living cells, causing radiation poisoning. The same is true for actinium.
Notes
- Ytterbite was named after the village it was discovered near, plus the -ite ending to indicate it was a mineral.
- Earths were given an -a ending and new elements are normally given an -ium ending.
- Unpenttrium, according to calculations, should have an electronic configuration of [Og]8s25g186f117d28p1/22.[37]
- For examples of this table see Atkins et al. (2006). Shriver & Atkins Inorganic Chemistry (4th ed.). Oxford: Oxford University Press • Myers et al. (2004). Holt Chemistry. Orlando: Holt, Rinehart & Winston • Chang R. (2000). Essential Chemistry (2nd ed.). Boston: McGraw-Hill
- For examples of the group 3 = Sc-Y-Lu-Lr table see Rayner-Canham G. & Overton T. (2013). Descriptive Inorganic Chemistry (6th ed.). New York: W. H. Freeman and Company • Brown et al. (2009). Chemistry: The Central Science (11th ed.). Upper Saddle River, New Jersey: Pearson Education • Moore et al. (1978). Chemistry. Tokyo: McGraw-Hill Kogakusha
- Notwithstanding, an IUPAC member subsequently wrote that, "IUPAC has not approved any specific form of the periodic table, and an IUPAC-approved form does not exist, though even members of IUPAC themselves have published diagrams titled “IUPAC Periodic Table of the Elements". However, the only specific recommendation IUPAC has made concerning the periodic table covers the Group numbering of 1–18."[105]
- For examples of the group 3 = Ln and An table see Housecroft C. E. & Sharpe A. G. (2008). Inorganic Chemistry (3rd ed.). Harlow: Pearson Education • Halliday et al. (2005). Fundamentals of Physics (7th ed.). Hoboken, NewJersey: John Wiley & Sons • Nebergall et al. (1980). General Chemistry (6th ed.). Lexington: D. C. Heath and Company
-
- 1922 Bohr's system, with bifurcations at Na, Mg, and Y
- 1939 Foster LS, [https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1056 "Why not modernise textbooks also? I. The periodic table", Journal of Chemical Education, vol. 16, no. 9. In group 3 the box under Y is “La {58–70}* Lu”, where * = the rare earths.
- 1947 Stedman's planar arrangement of his conic system
- 1952 Coryell, shows a step pyramid style table, with solid and dashed tie lines to show primary and secondary relationships. Just two elements are shown by him as having two solid tie lines: yttrium, to La-Ac and to Lu-Ac; and silicon, to Ti-Zr-Hf and to Ge-Sn-Pb.
- 1964 Sanderson RT, "A rational periodic table", Journal of Chemical Education, vol. 41, no. 4, pp. 187–189
- 1974 Mazurs EG, Graphic representations of the periodic system during one hundred years, The University of Alabama Press, Alabama, p. 77
- 2020 Vernon RE, "Organising the metals and nonmetals", Foundations of Chemistry, open access, see Electronic supplementary material
References
- van der Krogt, Peter. "39 Yttrium – Elementymology & Elements Multidict". Elements.vanderkrogt.net. Retrieved 2008-08-06.
- Emsley 2001, p. 496
- Gadolin, Johan (1794). "Undersökning af en svart tung Stenart ifrån Ytterby Stenbrott i Roslagen". Kongl. Vetenskaps Academiens Nya Handlingar (in Swedish). 15: 137–155.
- Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. p. 944. ISBN 0-7506-3365-4.
- Coplen, Tyler B.; Peiser, H. S. (1998). "History of the Recommended Atomic-Weight Values from 1882 to 1997: A Comparison of Differences from Current Values to the Estimated Uncertainties of Earlier Values (Technical Report)". Pure Appl. Chem. IUPAC's Inorganic Chemistry Division Commission on Atomic Weights and Isotopic Abundances. 70 (1): 237–257. doi:10.1351/pac199870010237.
- Heiserman, David L. (1992). "Element 39: Yttrium". Exploring Chemical Elements and their Compounds. New York: TAB Books. pp. 150–152. ISBN 0-8306-3018-X.
- Wöhler, Friedrich (1828). "Über das Beryllium und Yttrium". Annalen der Physik (in German). 89 (8): 577–582. Bibcode:1828AnP....89..577W. doi:10.1002/andp.18280890805.
- Ball, Philip (2002). The Ingredients: A Guided Tour of the Elements. Oxford University Press. pp. 100–102. ISBN 0-19-284100-9.
- Nilson, Lars Fredrik (1879). "Sur l'ytterbine, terre nouvelle de M. Marignac". Comptes Rendus (in French). 88: 642–647.
- Nilson, Lars Fredrik (1879). "Ueber Scandium, ein neues Erdmetall". Berichte der deutschen chemischen Gesellschaft (in German). 12 (1): 554–557. doi:10.1002/cber.187901201157.
- Cleve, Per Teodor (1879). "Sur le scandium". Comptes Rendus (in French). 89: 419–422.
- Fischer, Werner; Brünger, Karl; Grieneisen, Hans (1937). "Über das metallische Scandium". Zeitschrift für anorganische und allgemeine Chemie (in German). 231 (1–2): 54–62. doi:10.1002/zaac.19372310107.
- "The Discovery and Naming of the Rare Earths". Elements.vanderkrogt.net. Retrieved 23 June 2016.
- Greenwood and Earnshaw, p. 1424
- Weeks, Mary Elvira (1932). "The Discovery of the Elements: XI. Some Elements Isolated with the Aid of Potassium and Sodium:Zirconium, Titanium, Cerium and Thorium". The Journal of Chemical Education. 9 (7): 1231–1243. Bibcode:1932JChEd...9.1231W. doi:10.1021/ed009p1231.
- See:
- (Berzelius) (1839) "Nouveau métal" (New metal), Comptes rendus, 8 : 356–357. From p. 356: "L'oxide de cérium, extrait de la cérite par la procédé ordinaire, contient à peu près les deux cinquièmes de son poids de l'oxide du nouveau métal qui ne change que peu les propriétés du cérium, et qui s'y tient pour ainsi dire caché. Cette raison a engagé M. Mosander à donner au nouveau métal le nom de Lantane." (The oxide of cerium, extracted from cerite by the usual procedure, contains almost two fifths of its weight in the oxide of the new metal, which differs only slightly from the properties of cerium, and which is held in it so to speak "hidden". This reason motivated Mr. Mosander to give to the new metal the name Lantane.)
- (Berzelius) (1839) "Latanium — a new metal," Philosophical Magazine, new series, 14 : 390–391.
- Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. pp. 444–446. ISBN 0-07-049439-8. Retrieved 2009-06-06.
- Urbain, M. G. (1908). "Un nouvel élément, le lutécium, résultant du dédoublement de l'ytterbium de Marignac". Comptes rendus (in French). 145: 759–762.
- "Separation of Rare Earth Elements by Charles James". National Historic Chemical Landmarks. American Chemical Society. Retrieved 2014-02-21.
- von Welsbach; Carl Auer (1908). "Die Zerlegung des Ytterbiums in seine Elemente". Monatshefte für Chemie (in German). 29 (2): 181–225. doi:10.1007/BF01558944.
- Urbain, G. (1909). "Lutetium und Neoytterbium oder Cassiopeium und Aldebaranium – Erwiderung auf den Artikel des Herrn Auer v. Welsbach". Monatshefte für Chemie (in German). 31 (10): I. doi:10.1007/BF01530262.
- Clarke, F. W.; Ostwald, W.; Thorpe, T. E.; Urbain, G. (1909). "Bericht des Internationalen Atomgewichts-Ausschusses für 1909". Berichte der Deutschen Chemischen Gesellschaft (in German). 42 (1): 11–17. doi:10.1002/cber.19090420104.
- van der Krogt, Peter. "70. Ytterbium – Elementymology & Elements Multidict". Elements.vanderkrogt.net. Retrieved 4 July 2011.
- van der Krogt, Peter. "71. Lutetium – Elementymology & Elements Multidict". Elements.vanderkrogt.net. Retrieved 4 July 2011.
- Emsley, John (2001). Nature's building blocks: an A-Z guide to the elements. US: Oxford University Press. pp. 240–242. ISBN 0-19-850341-5.
- Debierne, André-Louis (1899). "Sur un nouvelle matière radio-active". Comptes rendus (in French). 129: 593–595.
- Debierne, André-Louis (1900–1901). "Sur un nouvelle matière radio-actif – l'actinium". Comptes rendus (in French). 130: 906–908.
- Giesel, Friedrich Oskar (1902). "Ueber Radium und radioactive Stoffe". Berichte der Deutschen Chemischen Gesellschaft (in German). 35 (3): 3608–3611. doi:10.1002/cber.190203503187.
- Giesel, Friedrich Oskar (1904). "Ueber den Emanationskörper (Emanium)". Berichte der Deutschen Chemischen Gesellschaft (in German). 37 (2): 1696–1699. doi:10.1002/cber.19040370280.
- Debierne, André-Louis (1904). "Sur l'actinium". Comptes rendus (in French). 139: 538–540.
- Giesel, Friedrich Oskar (1904). "Ueber Emanium". Berichte der Deutschen Chemischen Gesellschaft (in German). 37 (2): 1696–1699. doi:10.1002/cber.19040370280.
- Giesel, Friedrich Oskar (1905). "Ueber Emanium". Berichte der Deutschen Chemischen Gesellschaft (in German). 38 (1): 775–778. doi:10.1002/cber.190503801130.
- Ghiorso, Albert; Sikkeland, T.; Larsh, A. E.; Latimer, R. M. (1961). "New Element, Lawrencium, Atomic Number 103" (PDF). Phys. Rev. Lett. 6 (9): 473. Bibcode:1961PhRvL...6..473G. doi:10.1103/PhysRevLett.6.473.
- Flerov, G. N. (1967). "On the nuclear properties of the isotopes 256103 and 257103". Nucl. Phys. A. 106 (2): 476. Bibcode:1967NuPhA.106..476F. doi:10.1016/0375-9474(67)90892-5.
- Donets, E. D.; Shchegolev, V. A.; Ermakov, V. A. (1965). "Synthesis of the isotope of element 103 (lawrencium) with mass number 256". Atomnaya Énergiya (in Russian). 19 (2): 109.
- Translated in Donets, E. D.; Shchegolev, V. A.; Ermakov, V. A. (1965). "Synthesis of the isotope of element 103 (lawrencium) with mass number 256". Soviet Atomic Energy. 19 (2): 109. doi:10.1007/BF01126414.
- Greenwood, Norman N. (1997). "Recent developments concerning the discovery of elements 101–111". Pure Appl. Chem. 69 (1): 179–184. doi:10.1351/pac199769010179.
- Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 1-4020-3555-1.
- Pyykkö, Pekka (2011). "A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics. 13 (1): 161–8. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377.
- Nefedov, V.I.; Trzhaskovskaya, M.B.; Yarzhemskii, V.G. (2006). "Electronic Configurations and the Periodic Table for Superheavy Elements" (PDF). Doklady Physical Chemistry. 408 (2): 149–151. doi:10.1134/S0012501606060029. ISSN 0012-5016.
- Hofmann, Sigurd (2002). On Beyond Uranium. Taylor & Francis. p. 105. ISBN 978-0-415-28496-7.
- Roberto, J. B. (31 March 2015). "Actinide Targets for Super-Heavy Element Research" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 28 April 2017.
- van der Krogt, Peter. "Elementymology & Elements Multidict". Elements.vanderkrogt.net. Retrieved 4 July 2011.
- Eliav, E.; Kaldor, U.; Ishikawa, Y. (1995). "Transition energies of ytterbium, lutetium, and lawrencium by the relativistic coupled-cluster method". Phys. Rev. A. 52 (1): 291–296. Bibcode:1995PhRvA..52..291E. doi:10.1103/PhysRevA.52.291. PMID 9912247.
- Zou, Yu; Froese, Fischer C. (2002). "Resonance Transition Energies and Oscillator Strengths in Lutetium and Lawrencium". Phys. Rev. Lett. 88 (18): 183001. Bibcode:2002PhRvL..88b3001M. doi:10.1103/PhysRevLett.88.023001. PMID 12005680.
- Corbett, J. D. (1981). "Extended metal-metal bonding in halides of the early transition metals". Acc. Chem. Res. 14 (8): 239–246. doi:10.1021/ar00068a003.
- Nikolai B., Mikheev; Auerman, L. N.; Rumer, Igor A.; Kamenskaya, Alla N.; Kazakevich, M. Z. (1992). "The anomalous stabilisation of the oxidation state 2+ of lanthanides and actinides". Russian Chemical Reviews. 61 (10): 990–998. Bibcode:1992RuCRv..61..990M. doi:10.1070/RC1992v061n10ABEH001011.
- Kang, Weekyung; Bernstein, E. R. (2005). "Formation of Yttrium Oxide Clusters Using Pulsed Laser Vaporization". Bull. Korean Chem. Soc. 26 (2): 345–348. doi:10.5012/bkcs.2005.26.2.345.
- Cotton, S. A. (1994). "Scandium, Yttrium and the Lanthanides: Inorganic and Coordination Chemistry". Encyclopedia of Inorganic Chemistry. John Wiley & Sons. ISBN 0-471-93620-0.
- Dean, John A. (1999). Lange's handbook of chemistry (Fifteenth ed.). McGraw-Hill, Inc. pp. 589–592. ISBN 0-07-016190-9.
- Barbalace, Kenneth. "Periodic Table of Elements Sorted by Melting Point". Environmental Chemistry.com. Retrieved 2011-05-18.
- Barbalace, Kenneth. "Periodic Table of Elements Sorted by Boiling Point". Environmental Chemistry.com. Retrieved 2011-05-18.
- Fournier, Jean-Marc (1976). "Bonding and the electronic structure of the actinide metals". Journal of Physics and Chemistry of Solids. 37 (2): 235–244. Bibcode:1976JPCS...37..235F. doi:10.1016/0022-3697(76)90167-0.
- Penneman, R. A.; Mann, J. B. (1976). "'Calculation chemistry' of the superheavy elements; comparison with elements of the 7th period". Proceedings of the Moscow Symposium on the Chemistry of Transuranium Elements: 257–263. doi:10.1016/B978-0-08-020638-7.50053-1.
- Lide, D. R., ed. (2003). CRC Handbook of Chemistry and Physics (84th ed.). Boca Raton, FL: CRC Press.
- Barbalace, Kenneth. "Scandium". Chemical Book. Retrieved 2011-05-18.
- Barbalace, Kenneth. "Yttrium". Chemical Book. Retrieved 2011-05-18.
- Thyssen, P.; Binnemans, K. (2011). Gschneidner Jr., K. A.; Bünzli, J-C.G; Vecharsky, Bünzli (eds.). Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis. Handbook on the Physics and Chemistry of Rare Earths. 41. Amsterdam: Elsevier. pp. 1–94. doi:10.1016/B978-0-444-53590-0.00001-7. ISBN 978-0-444-53590-0.
- Thyssen P. and Binnemans K. (2011). "Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis". In K. A. Gschneider Jr. (ed). Handbook on the Physics and Chemistry of the Rare Earths. 41. Amsterdam: Elsevier, pp. 1–94; Seaborg G. T. (1994). Origin of the Actinide Concept'. In K. A. Gschneider Jr. (ed). Handbook on the Physics and Chemistry of the Rare Earths. 18. Amsterdam: Elsevier, pp. 1–27.
- Stewart, P. J. (2008). "The Flyleaf Table: An Alternative". Journal of Chemical Education. 85 (11): 1490. Bibcode:2008JChEd..85.1490S. doi:10.1021/ed085p1490.
- Jørgensen, Christian K. (1988). "Influence of rare earths on chemical understanding and classification". Handbook on the Physics and Chemistry of Rare Earths. 11. pp. 197–292. doi:10.1016/S0168-1273(88)11007-6. ISBN 9780444870803.
- Thyssen, P.; Binnemanns, K. (2011). "1: Accommodation of the rare earths in the periodic table: A historical analysis". In Gschneidner Jr., K. A.; Büzli, J-C. J.; Pecharsky, V. K. (eds.). Handbook on the Physics and Chemistry of Rare Earths. 41. Amsterdam: Elsevier. pp. 80–81. ISBN 978-0-444-53590-0.
- Keeler, J.; Wothers, P. (2014). Chemical Structure and Reactivity: An Integrated Approach. Oxford: Oxford University. p. 259. ISBN 978-0-19-960413-5.
- Scerri, E. (2012). "Mendeleev's Periodic Table Is Finally Completed and What To Do about Group 3?". Chemistry International. 34 (4). doi:10.1515/ci.2012.34.4.28. Archived from the original on 5 July 2017.
- Castelvecchi, D. (8 April 2015). "Exotic atom struggles to find its place in the periodic table". Nature. doi:10.1038/nature.2015.17275. Archived from the original on 5 October 2015. Retrieved 20 September 2015.
- "The constitution of group 3 of the periodic table". IUPAC. 2015. Archived from the original on 5 July 2016. Retrieved 30 July 2016.
- Emsley, J. (2011). Nature's Building Blocks (new ed.). Oxford: Oxford University. p. 651. ISBN 978-0-19-960563-7.
- William B. Jensen (1982). "The Positions of Lanthanum (Actinium) and Lutetium (Lawrencium) in the Periodic Table". J. Chem. Educ. 59 (8): 634–636. Bibcode:1982JChEd..59..634J. doi:10.1021/ed059p634.
- Trifonov, D. N. (1970). Rare-earth elements and their position in the periodic system (translated from Russian). New Delhi: Indian National Scientific Documentation Centre. pp. 201–202.
- Scerri, Eric (2016). "The Changing Views of a Philosopher of Chemistry on the Question of Reduction". In Scerri, Eric; Fisher, Grant (eds.). Essays in the Philosophy of Chemistry. Oxford University Press.
- Schwarz, W. H. Eugen (2010). "The Full Story of the Electron Configurations of the Transition Elements". Journal of Chemical Education. 87 (4): 444–8. doi:10.1021/ed8001286.
- Jensen, William B. (2009). "Misapplying the Periodic Law" (PDF). Journal of Chemical Education. 86 (10): 1186. Bibcode:2009JChEd..86.1186J. doi:10.1021/ed086p1186. Retrieved 16 May 2020.
- Scerri, Eric (2009). "Which Elements Belong in Group 3?". Journal of Chemical Education. 86 (10): 1188. Bibcode:2009JChEd..86.1188S. doi:10.1021/ed086p1188.
- Greenwood, N. N.; Harrington, T. J. (1973). The chemistry of the transition elements. Oxford: Clarendon Press. p. 50. ISBN 978-0-19-855435-6.
- Aylward, G.; Findlay, T. (2008). SI chemical data (6th ed.). Milton, Queensland: John Wiley & Sons. ISBN 978-0-470-81638-7.
- Wiberg, N. (2001). Inorganic Chemistry. San Diego: Academic Press. p. 119. ISBN 978-0-12-352651-9.
- Wulfsberg, G. (2006). "Periodic table: Trends in the properties of the elements". Encyclopedia of Inorganic Chemistry. New York: John Wiley & Sons. p. 3. ISBN 978-0-470-86210-0.
- Cotton, S. (2007). Lanthanide and Actinide Chemistry. Chichester: John Wiley & Sons. p. 150. ISBN 978-0-470-01006-8.
- Jørgensen, Christian K. (1983). "5f Electrons in Transthorium Chemistry" (PDF). Radiochimica Acta. 32 (1–3): 1–5. doi:10.1524/ract.1983.32.13.1. Retrieved 16 May 2020.
- Seaborg, Glenn T. (August 1991). "Origin of the Actinide Concept" (PDF). escholarship.org. Retrieved 17 May 2020.
- Rayner-Canham, Geoff (2013). "Periodic patterns: the Group (n) and Group (n + 10) linkage". Foundations of Chemistry. 15 (2): 229–237. doi:10.1007/s10698-012-9169-6.
- Jensen, William B. (August 2003). "The Place of Zinc, Cadmium, and Mercury in the Periodic Table" (PDF). Journal of Chemical Education. 80 (8): 952–961. doi:10.1021/ed080p952. Retrieved 17 May 2020.
- Moeller et al. (1989). Chemistry with Inorganic Qualitative Analysis (3rd ed.). SanDiego: Harcourt Brace Jovanovich, pp. 955–956, 958.
- L. D. Landau, E. M. Lifshitz (1958). Quantum Mechanics: Non-Relativistic Theory. Vol. 3 (1st ed.). Pergamon Press. pp. 256–7.
- Scerri, E. (15 September 2015). "Five ideas in chemical education that must die – Group three". Education in Chemistry. Royal Society of Chemistry. Archived from the original on 23 December 2015. Retrieved 19 September 2015.
It is high time that the idea of group 3 consisting of Sc, Y, La and Ac is abandoned
- Hamilton, David C. (1965). "Position of Lanthanum in the Periodic Table". American Journal of Physics. 33 (8): 637–640. doi:10.1119/1.1972042.
- Xu, Wei; Ji, Wen-Xin; Qiu, Yi-Xiang; Schwarz, W. H. Eugen; Wang, Shu-Guang (2013). "On structure and bonding of lanthanoid trifluorides LnF3 (Ln = La to Lu)". Physical Chemistry Chemical Physics. 2013 (15): 7839–47. Bibcode:2013PCCP...15.7839X. doi:10.1039/C3CP50717C. PMID 23598823.
- Wittig, Jörg (1973). "The pressure variable in solid state physics: What about 4f-band superconductors?". In H. J. Queisser (ed.). Festkörper Probleme: Plenary Lectures of the Divisions Semiconductor Physics, Surface Physics, Low Temperature Physics, High Polymers, Thermodynamics and Statistical Mechanics, of the German Physical Society, Münster, March 19–24, 1973. Advances in Solid State Physics. 13. Berlin, Heidelberg: Springer. p. 375–396. doi:10.1007/BFb0108579. ISBN 978-3-528-08019-8.
- Östlin, A. (2013). "Transition metals" (PDF). Electronic Structure Studies and Method Development for Complex Materials (Licentiate). p. 13. Retrieved 17 May 2020.
- Gschneidner Jr., K. A. (December 1971). "On the nature of 4ƒ bonding in the lanthanide elements and their compounds". Journal of the Less Common Metals. 25 (4): 405–422. doi:10.1016/0022-5088(71)90184-6.
- Glotzel, D. (1978). "Ground-state properties of f band metals: lanthanum, cerium and thorium". Journal of Physics F: Metal Physics. 8 (7): L163–L168. Bibcode:1978JPhF....8L.163G. doi:10.1088/0305-4608/8/7/004.
- Alvarez, Santiago (11 February 2020). "The transition from 4f to 5d elements from the structural point of view". CrystEngComm. 2020 (Advance Article). doi:10.1039/D0CE00029A.
- Merz, H.; Ulmer, K. (1967). "Position of Lanthanum and Lutetium in the Periodic Table". Physics Letters A. 26 (1): 6–7. Bibcode:1967PhLA...26....6M. doi:10.1016/0375-9601(67)90527-0.
- Jensen, W. B. (2015). "Some Comments on the Position of Lawrencium in the Periodic Table" (PDF). Archived from the original (PDF) on 23 December 2015. Retrieved 20 September 2015.
- Xu, W-H.; Pyykkö, P. (2016). "Is the chemistry of lawrencium peculiar?" (PDF). Physical Chemistry Chemical Physics. 18 (26): 17351–17355. Bibcode:2016PCCP...1817351X. doi:10.1039/C6CP02706G. hdl:10138/224395. PMID 27314425.
- Silva, Robert J. (2006). "Fermium, Mendelevium, Nobelium, and Lawrencium" (PDF). In Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements. 3 (3rd ed.). Dordrecht: Springer. pp. 1621–1651. doi:10.1007/1-4020-3598-5_13. ISBN 978-1-4020-3555-5. Archived from the original (PDF) on 2010-07-17.
- Iyakutti, K.; Dakshinamoorthy, M.; Asokamani, R. (1981). "Bandstructure of actinium and the effect of correlation". Solid State Communications. 40 (5): 555–7. doi:10.1016/0038-1098(81)90572-X.
- Dakshinamoorthy, M.; Iyakutti, K. (1984). "Band structure, Fermi surface, superconductivity, and resistivity of actinium under high pressure". Physical Review B. 30 (12): 6943–50. doi:10.1103/PhysRevB.30.6943.
- King, R. B. (1995). Inorganic Chemistry of Main Group Elements. New York: Wiley-VCH. p. 289. ISBN 978-1-56081-679-9.
- Greenwood and Earnshaw, p. 958
- Greenwood and Earnshaw, p. 947
- Greenwood and Earnshaw, p. 957
- Connelly, N. G.; Damhus, T.; Hartshorn, R. M.; Hutton, A. T. (2005). Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005 (PDF). RSC Publishing. p. vii. ISBN 978-0-85404-438-2. Archived (PDF) from the original on 23 November 2018. Retrieved 26 November 2018.
Lesser omissions include ... the several different outdated versions of the periodic table. (That on the inside front cover is the current IUPAC-agreed version.)
- Leigh, G. J. (2009). "Periodic Tables and IUPAC". Chemistry International. 31 (1). doi:10.1515/ci.2009.31.1.4. Archived from the original on 27 November 2018. Retrieved 27 November 2018.
- Jensen, William B. (2008). "The Periodic Table: Facts or Committees?". Journal of Chemical Education. 85 (11): 1491–2. Bibcode:2008JChEd..85.1491J. doi:10.1021/ed085p1491.2.
- Scerri, P.; Parsons, B. (2018). "What elements belong in group 3 of the Periodic Table?". In Scerri, E.; Restrepo, G. (eds.). From Mendeleev to Oganesson: A Multidisciplinary Perspective on the Periodic Table. New York: Oxford University Press. pp. 140–151. ISBN 978-0-190-66853-2.
- Lee, J. D. (1996). Concise inorganic chemistry (5th ed.). Oxford: Blackwell-Science. p. 679. ISBN 978-0-6320-5293-6.
- Barbalace, Kenneth. "Periodic Table of Elements". Environmental Chemistry.com. Retrieved 2007-04-14.
- Bernhard, F. (2001). "Scandium mineralization associated with hydrothermal lazurite-quartz veins in the Lower Austroalpie Grobgneis complex, East Alps, Austria". Mineral Deposits in the Beginning of the 21st Century. Lisse: Balkema. ISBN 90-265-1846-3.
- Kristiansen, Roy (2003). "Scandium – Mineraler I Norge" (PDF). Stein (in Norwegian): 14–23. Archived from the original (PDF) on October 8, 2010.
- von Knorring, O.; Condliffe, E. (1987). "Mineralized pegmatites in Africa". Geological Journal. 22: 253. doi:10.1002/gj.3350220619.
- Stwertka, Albert (1998). "Yttrium". Guide to the Elements (Revised ed.). Oxford University Press. pp. 115–116. ISBN 0-19-508083-1.
- Hedrick, James B. "Rare-Earth Metals" (PDF). USGS. Retrieved 2009-06-06.
- Castor, Stephen B.; Hedrick, James B. "Rare Earth Elements" (PDF). Retrieved 2009-06-06.
- "Mineral Commodity Summaries 2010: Yttrium" (PDF). United States Geological Survey. Retrieved 2011-07-07.
- Emsley 2001, p. 241
- Deschamps, Y. "Scandium" (PDF). mineralinfo.com. Archived from the original (PDF) on February 25, 2009. Retrieved 2008-10-21.
- "Mineral Commodity Summaries 2010: Scandium" (PDF). United States Geological Survey. Retrieved 2011-07-07.
- Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1056–1057. ISBN 3-11-007511-3.
- Lenntech (1998). "Scandium (Sc) — chemical properties of scandium, health effects of scandium, environmental effects of scandium". Lenntech. Retrieved 2011-05-21.
- MacDonald, N. S.; Nusbaum, R. E.; Alexander, G. V. (1952). "The Skeletal Deposition of Yttrium" (PDF). Journal of Biological Chemistry. 195 (2): 837–841. PMID 14946195.
- Emsley 2001, pp. 495–498
- Emsley 2001, p. 240
- Pol, Arjan; Barends, Thomas R. M.; Dietl, Andreas; Khadem, Ahmad F.; Eygensteyn, Jelle; Jetten, Mike S. M.; Op Den Camp, Huub J. M. (2013). "Rare earth metals are essential for methanotrophic life in volcanic mudpots". Environmental Microbiology. 16 (1): 255–64. doi:10.1111/1462-2920.12249. PMID 24034209.
Bibliography
- Emsley, John (2001). Nature's building blocks: an A-Z guide to the elements. US: Oxford University Press. ISBN 0-19-850341-5.