Synthetic element
A synthetic element is one of 24 chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table:[1] these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons onto the nucleus of an element with an atomic number lower than 95. All synthetic elements are unstable, but they decay at a widely varying rate: their half-lives range from 15.6 million years to a few hundred microseconds.
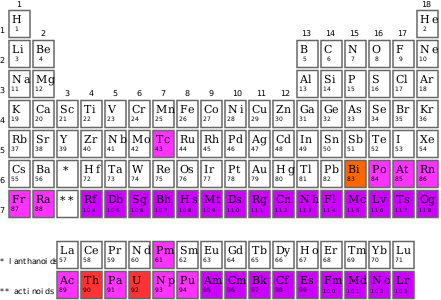
Seven other elements that were created artificially—and thus initially considered to be synthetic—were later discovered to exist in nature in trace quantities. The first, technetium, was created in 1937.[2] Plutonium, atomic number 94, first synthesized in 1940, is another such element. It is the element with the largest number of protons (and equivalent atomic number) to occur in nature, but it does so in such tiny quantities that it is far more practical to synthesize it. Plutonium is extremely well-known due to its use in atomic bombs and nuclear reactors.[3] No elements with an atomic number greater than 99 have any uses outside of scientific research, since they have extremely short half-lives, and thus have never been produced in large quantities.
Properties
Any elements with atomic number greater than 94 present at the formation of the earth about 4.6 billion years ago have decayed sufficiently rapidly into lighter elements relative to the age of Earth that any atoms of these elements that may have existed when the Earth formed have long since decayed.[4][5] Atoms of synthetic elements now present on Earth are the product of atomic bombs or experiments that involve nuclear reactors or particle accelerators, via nuclear fusion or neutron absorption.[6]
Atomic mass for natural elements is based on weighted average abundance of natural isotopes that occur in Earth's crust and atmosphere. For synthetic elements, the isotope depends on the means of synthesis, so the concept of natural isotope abundance has no meaning. Therefore, for synthetic elements the total nucleon count (protons plus neutrons) of the most stable isotope, i.e. the isotope with the longest half-life—is listed in brackets as the atomic mass.
History
Technetium
The first element discovered through synthesis was technetium—its discovery being definitely confirmed in 1937.[7] This discovery filled a gap in the periodic table, and the fact that no stable isotopes of technetium exist explains its natural absence on Earth (and the gap).[8] With the longest-lived isotope of technetium, 97Tc, having a 4.21-million-year half-life,[9] no technetium remains from the formation of the Earth.[10][11] Only minute traces of technetium occur naturally in the Earth's crust—as a spontaneous fission product of uranium-238 or by neutron capture in molybdenum ores—but technetium is present naturally in red giant stars.[12][13][14][15]
Curium
The first discovered purely synthetic element was curium, synthesized in 1944 by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso by bombarding plutonium with alpha particles.[16][17][18][19]
Eight others
The discoveries of americium, berkelium, and californium followed soon. Einsteinium and fermium were discovered by a team of scientists led by Albert Ghiorso in 1952 while studying the radioactive debris from the detonation of the first hydrogen bomb.[20] The isotopes discovered were einsteinium-253, with a half-life of 20.5 days, and fermium-255, with a half-life of about 20 hours. The discoveries of mendelevium, nobelium, and lawrencium followed.
Rutherfordium and dubnium
During the height of the Cold War, teams from the Soviet Union and the United States independently discovered rutherfordium and dubnium. The naming and credit for discovery of these elements remained unresolved for many years, but eventually shared credit was recognized by IUPAC/IUPAP in 1992. In 1997, IUPAC decided to give dubnium its current name honoring the city of Dubna where the Russian team made their discoveries since American-chosen names had already been used for many existing synthetic elements, while the name rutherfordium (chosen by the American team) was accepted for element 104.
The last thirteen
Meanwhile, the American team had discovered seaborgium, and the next six elements had been discovered by a German team: bohrium, hassium, meitnerium, darmstadtium, roentgenium, and copernicium. Element 113, nihonium, was discovered by a Japanese team; the last five known elements, flerovium, moscovium, livermorium, tennessine, and oganesson, were discovered by Russian–American collaborations and complete the seventh row of the periodic table.
List of synthetic elements
The following elements do not occur naturally on Earth. All are transuranium elements and have atomic numbers of 95 and higher.
| Element name | Chemical Symbol | Atomic Number | First definite synthesis |
|---|---|---|---|
| Americium | Am | 95 | 1944 |
| Curium | Cm | 96 | 1944 |
| Berkelium | Bk | 97 | 1949 |
| Californium | Cf | 98 | 1950 |
| Einsteinium | Es | 99 | 1952 |
| Fermium | Fm | 100 | 1952 |
| Mendelevium | Md | 101 | 1955 |
| Lawrencium | Lr | 103 | 1961 |
| Nobelium | No | 102 | 1966 |
| Rutherfordium | Rf | 104 | 1966 (USSR), 1969 (US) * |
| Dubnium | Db | 105 | 1968 (USSR), 1970 (US) * |
| Seaborgium | Sg | 106 | 1974 |
| Bohrium | Bh | 107 | 1981 |
| Hassium | Hs | 108 | 1984 |
| Meitnerium | Mt | 109 | 1982 |
| Darmstadtium | Ds | 110 | 1994 |
| Roentgenium | Rg | 111 | 1994 |
| Copernicium | Cn | 112 | 1996 |
| Nihonium | Nh | 113 | 2003–4 |
| Flerovium | Fl | 114 | 1999 |
| Moscovium | Mc | 115 | 2003 |
| Livermorium | Lv | 116 | 2000 |
| Tennessine | Ts | 117 | 2010 |
| Oganesson | Og | 118 | 2002 |
| * Shared credit for discovery. | |||
Other elements usually produced through synthesis
All elements with atomic numbers 1 through 94 occur naturally at least in trace quantities, but the following elements are often produced through synthesis. Technetium, promethium, astatine, neptunium, and plutonium were discovered through synthesis before being found in nature.
| Element name | Chemical Symbol | Atomic Number | First definite discovery |
|---|---|---|---|
| Technetium | Tc | 43 | 1937 |
| Promethium | Pm | 61 | 1945 |
| Polonium | Po | 84 | 1898 |
| Astatine | At | 85 | 1940 |
| Francium | Fr | 87 | 1939 |
| Actinium | Ac | 89 | 1902 |
| Protactinium | Pa | 91 | 1913 |
| Neptunium | Np | 93 | 1940 |
| Plutonium | Pu | 94 | 1940 |
References
- Kulkarni, Mayuri. "A Complete List of Man-made Synthetic Elements". ScienceStuck. Retrieved 15 May 2019.
- "WebElements Periodic Table » Technetium » historical information". www.webelements.com. Webelements. Retrieved 7 November 2019.
- Bradford, Alina. "Facts About Plutonium". LiveSci=nce. Retrieved 16 May 2019.
- Redd, Nola. "How Was Earth Formed?". Space.com. Retrieved 16 May 2019.
- "Synthetic elements". Infoplease. Retrieved 16 May 2019.
- Kulkarni, Mayuri. "A Complete List of Man-made Synthetic Elements". ScienceStuck. Retrieved 16 May 2019.
- Helmenstine, Anne Marie. "Technetium or Masurium Facts". ThoughtCo. ThoughtCo. Retrieved 15 May 2019.
- "Technetium decay and its cardiac application". KhanAcademy. Khan Academy. Retrieved 15 May 2019.
- Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- Stewart, Doug. "Technetium Element Facts". Chemicool. Retrieved 15 May 2019.
- Yinon, Yinon. "Periodic Table: Technetium". Chemical Elements. Retrieved 15 May 2019.
- Hammond, C. R. (2004). "The Elements". Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 978-0-8493-0485-9.
- Moore, C. E. (1951). "Technetium in the Sun". Science. 114 (2951): 59–61. Bibcode:1951Sci...114...59M. doi:10.1126/science.114.2951.59. PMID 17782983.
- Dixon, P.; Curtis, David B.; Musgrave, John; Roensch, Fred; Roach, Jeff; Rokop, Don (1997). "Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials". Analytical Chemistry. 69 (9): 1692–9. doi:10.1021/ac961159q. PMID 21639292.
- Curtis, D.; Fabryka-Martin, June; Dixon, Paul; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta. 63 (2): 275. Bibcode:1999GeCoA..63..275C. doi:10.1016/S0016-7037(98)00282-8.
- Krebs, Robert E. The history and use of our earth's chemical elements: a reference guide, Greenwood Publishing Group, 2006, ISBN 0-313-33438-2 p. 322
- Harper, Douglas. "pandemonium". Online Etymology Dictionary.
- Harper, Douglas. "delirium". Online Etymology Dictionary.
- Hall, Nina (2000). The New Chemistry: A Showcase for Modern Chemistry and Its Applications. Cambridge University Press. pp. 8–9. ISBN 978-0-521-45224-3.
- Ghiorso, Albert (2003). "Einsteinium and Fermium". Chemical and Engineering News. 81 (36): 174–175. doi:10.1021/cen-v081n036.p174.
External links
- "einsteinium (Es) - chemical element". Britannica.com. Retrieved 23 May 2017.
- "mendelevium (Md) - chemical element". Britannica.com. Retrieved 23 May 2017.
- "synthetic elements". Encyclopedia2.thefreedictionary.com. Retrieved 23 May 2017.
- "It's Elemental - The Element Fermium". Education.jlab.org. Retrieved 23 May 2017.
- Kulkarni, Mayuri. "A Complete List of Man-made Synthetic Elements". ScienceStuck. Retrieved 15 May 2019.