Gallium antimonide
Gallium antimonide (GaSb) is a semiconducting compound of gallium and antimony of the III-V family. It has a lattice constant of about 0.61 nm. It has a band gap of 0.67 eV.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Gallium(III) antimonide | |
| Other names
Gallium antimonide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.859 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| GaSb | |
| Molar mass | 191.483 g/mol |
| Density | 5.614 g/cm3 |
| Melting point | 712 °C (1,314 °F; 985 K) |
| insoluble | |
| Band gap | 0.726 eV (300 K) |
| Electron mobility | 3000 cm2/(V*s) (300 K) |
| Thermal conductivity | 0.32 W/(cm*K) (300 K) |
Refractive index (nD) |
3.8 |
| Structure | |
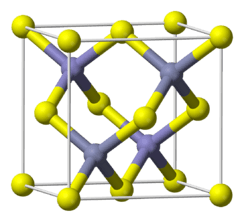
| Sphalerite, cF8 | |
| F-43m, No. 216 | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Gallium nitride Gallium phosphide Gallium arsenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
The intermetallic compound GaSb was first prepared in 1926 by Victor Goldschmidt, who directly combined the elements under an inert gas atmosphere and reported on GaSb's lattice constant, which has since been revised. Goldschmidt also synthesized gallium phosphide and gallium arsenide.[2] The Ga-Sb phase equilibria was investigated in 1955 by Koster[3] and by Greenfield.[4]
Applications
GaSb can be used for Infrared detectors, infrared LEDs and lasers and transistors, and thermophotovoltaic systems.
References
- Dubey, S.K.; Dubey, R.L.; Yadav, A.D.; Jadhav, V.; Rao, T.K. Gundu; Mohanty, T.; Kanjilal, D. (2006). "Study of optical properties of swift heavy ion irradiated gallium antimonide". Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 244 (1): 141–144. doi:10.1016/j.nimb.2005.11.131. ISSN 0168-583X.
- Goldschmidt, V. M., Skr. Akad. Oslo, 8 (1926).
- Koster, W.; Thoma, B., Z. Metallkd. 46, 291 (1955).
- Greenfield, I. G.; Smith, R. L., Trans. AIME 203, 351 (1955).
External links
- properties listed at NSM, Ioffe Institute.
- National Compound Semiconductor Roadmap at the Office of Naval Research
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
