Flupropadine
Flupropadine is a rodenticide.[1][2] It was sold under the trade name Rhone Poulenc.[3] Originally made by May and Baker[4] and tested on farms in the United Kingdom it was withdrawn from use by 1994.[5] Flupropadine has a delayed action, and so rodents can have multiple feeds from the bait before being killed.[6]
 | |
| Names | |
|---|---|
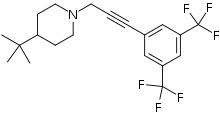
| IUPAC name
1-{3-[3,5-Bis(trifluoromethyl)phenyl]-2-propyn-1-yl}-4-(2-methyl-2-propanyl)piperidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H23F6N | |
| Molar mass | 391.401 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The molecule has two rings, one is a m-hexafluoroxylene, and the other is piperidine. Flupropadine is made from 3,5-bis(trifluoromethyl)iodobenzene, propargyl alcohol, and 4-tert-butylpiperidine.[3]
References
- Buckle, A. P (1985). "Field trials of a new sub-acute rodenticide flupropadine, against wild Norway rats (Rattus norvegicus)". The Journal of Hygiene. 95 (2): 505–12. doi:10.1017/s0022172400062926. PMC 2129537. PMID 3840823.
- Rowe, F. P; Bradfield, A; Swinney, T (1985). "Pen and field trials of flupropadine against the house mouse (Mus musculus L.)". The Journal of Hygiene. 95 (2): 513–8. doi:10.1017/s0022172400062938. PMC 2129553. PMID 4067302.
- Unger, Thomas A. (1996). Pesticide Synthesis Handbook. William Andrew. pp. 499–500. ISBN 9780815518532.
- Missio, Andrea (14 June 2006). "Hexafluoroxylenes: Fluorine Chemistry and Beyond" (PDF). p. 7.
- Berny, Philipe (May 2003). "STATE-OF-THE-ART REPORT ON THE USE OF ANTICOAGULANT RODENTICIDES IN THE EU AND BEYOND". Communication and Information Resource Centre for Administrations, Businesses and Citizens. Retrieved 15 May 2018.
- Buckle, Alan P.; Smith, Robert H. (2015). Rodent Pests and Their Control, 2nd Edition. CABI. p. 116. ISBN 9781845938178.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.