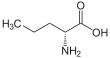
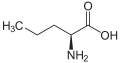
Norvaline
Norvaline (abbreviated as Nva) is an amino acid with the formula CH3(CH2)2CH(NH2)CO2H. The compound is an isomer of the more common amino acid valine.[2] Like most other α-amino acids, norvaline is chiral. It is a white, water-soluble solid.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Aminopentanoic acid | |||
| Other names
2-Aminovaleric acid; α-Aminopentanoic acid; Propylglycine | |||
| Identifiers | |||
3D model (JSmol) |
| ||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.026.858 | ||
PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C5H11NO2 | |||
| Molar mass | 117.148 g·mol−1 | ||
| Acidity (pKa) | 2.36 (carboxyl), 9.76 (amino)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Occurrence
Norvaline is a non-proteinogenic unbranched-chain amino acid. It has previously been reported to be a natural component of an antifungal peptide of Bacillus subtilis. Norvaline and other modified unbranched chain amino acids have received attention because they appear to be incorporated in some recombinant proteins found in E. coli.[3] Its biosynthesis has been examined. The incorporation of Nva into peptides reflects the imperfect selectivity of the associated aminoacyl-tRNA synthetase. In Miller–Urey experiments probing prebiotic synthesis of amino acids, norvaline, but also norleucine, are produced.[4]
Norvaline is known to promote tissue regeneration and muscle growth,[5] and to become a precursor in the penicillin biosynthetic pathway.[6][7]
Norvaline has not been reported to be toxic at doses up to 5 grams a day, and it is commonly used by bodybuilders to increase muscle mass. At higher concentration, over 5 grams a day, norvaline was shown to decrease cell viability (cell concentration 125 um) and caused necrotic cell death and significant changes to mitochondrial morphology and function. At high concentrations, all amino acids are cytotoxic, and norvaline toxicity could be attributed to protein amino acid mimicry.[8] However, the conclusions of this study have been recently questioned, because the demonstrated l-norvaline toxicity is limited to specific in vitro assays at exceedingly high concentrations. [9] Moreover, in higher organisms, l-norvaline is well tolerated, and in vivo toxicity is not apparent.[10]
Nomenclature
Norvaline and norleucine (one hydrocarbon group longer) both possess the nor- prefix for historical reason, despite current conventional usage of the prefix to denote a missing hydrocarbon group (under which they would theoretically be called "dihomoalanine" and "trihomoalanine"). The name is not systematic, and the IUPAC/IUB Joint Commission on Nomenclature recommends that this name should be abandoned and the systematic name should be used.[11]
Use in treatment of Alzheimer's disease
Norvaline is a promising candidate drug for the treatment of Alzheimer's disease. It is a non-competitive arginase inhibitor which readily crosses the blood-brain barrier, and reduces arginine loss in the brain. Amyloid-beta deposition is associated with L-arginine deprivation and neurodegeneration. Mice treated with Norvaline display improved spatial memory, increased neuroplasticity-related proteins, and decrease in amyloid-beta.[12]
References
- Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- Merriam-Webster Retrieved 4 September 2010
- Soini J, Falschlehner C, Liedert C, Bernhardt J, Vuoristo J, Neubauer P (2008). "Norvaline is accumulated after a down-shift of oxygen in Escherichia coli W3110". Microbial Cell Factories. 7: 30. doi:10.1186/1475-2859-7-30. PMC 2579280. PMID 18940002.
- Alvarez-Carreño C, Becerra A, Lazcano A (October 2013). "Norvaline and norleucine may have been more abundant protein components during early stages of cell evolution". Origins of Life and Evolution of the Biosphere. 43 (4–5): 363–75. Bibcode:2013OLEB...43..363A. doi:10.1007/s11084-013-9344-3. PMID 24013929.
- Ming XF, Rajapakse AG, Carvas JM, Ruffieux J, Yang Z (2009). "Inhibition of S6K1 accounts partially for the anti-inflammatory effects of the arginase inhibitor L-norvaline". BMC Cardiovascular Disorders. 9: 12. doi:10.1186/1471-2261-9-12. PMC 2664787. PMID 19284655.
- reference.md Retrieved 4 September 2010
- Kisumi M, Sugiura M, Chibata I (August 1976). "Biosynthesis of norvaline, norleucine, and homoisoleucine in Serratia marcescens". Journal of Biochemistry. 80 (2): 333–9. doi:10.1093/oxfordjournals.jbchem.a131281. PMID 794063.
- Samardzic, Kate; Rodgers, Kenneth J. (2019-04-01). "Cytotoxicity and mitochondrial dysfunction caused by the dietary supplement l-norvaline". Toxicology in Vitro. 56: 163–171. doi:10.1016/j.tiv.2019.01.020. ISSN 0887-2333. PMID 30703532.
- Polis B, Gilinsky MA, Samson AO (2019-12-17). "Reports of L-Norvaline Toxicity in Humans May Be Greatly Overstated". Brain Sciences. 9: 382. doi:10.3390/brainsci9120382. ISSN 2076-3425. PMID 31861122.
- El-Bassossy HM, El-Fawal R, Fahmy A, Watson ML (2012-01-09). "Arginase inhibition alleviates hypertension in the metabolic syndrome". British Journal of Pharmacology. 169: 693–703. doi:10.1111/bph.12144. ISSN 1476-5381. PMC 3682715. PMID 23441715.
- "Nomenclature and Symbolism For Amino Acids and Peptides". Pure and Applied Chemistry. 56 (5): 595–624. 1984. doi:10.1351/pac198456050595.
- Polis B, Srikanth KD, Elliott E, Gil-Henn H, Samson AO (October 2018). "L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer's Disease". Neurotherapeutics. 15 (4): 1036–1054. doi:10.1007/s13311-018-0669-5. PMC 6277292. PMID 30288668.