Zinc nitrate
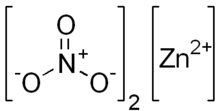 | |
| Names | |
|---|---|
| IUPAC name
Zinc nitrate | |
| Other names
Zinc Nitrate Hexahydrate(hexahydrate) | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.039 |
| EC Number | 231-943-8 |
PubChem CID |
|
| RTECS number | ZH4772000 |
| UN number | 1514 |
| |
| |
| Properties | |
| Zn(NO3)2 | |
| Molar mass | 189.36 g/mol (anhydrous) 297.49 g/mol (hexahydrate) |
| Appearance | colorless, deliquescent crystals |
| Density | 2.065 g/cm3 (hexahydrate) |
| Melting point | 110 °C (230 °F; 383 K) (anhydrous) 45.5 °C (trihydrate) 36.4 °C (hexahydrate) |
| Boiling point | ~ 125 °C (257 °F; 398 K) decomposes (hexahydrate) |
| 327 g/100 mL, 40 °C (trihydrate) 184.3 g/100 ml, 20 °C (hexahydrate) | |
| Solubility | very soluble in alcohol |
| −63.0·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Oxidant, may explode on heating |
| Safety data sheet | ICSC 1206 |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Zinc sulfate Zinc chloride |
Other cations |
Cadmium nitrate Mercury(II) nitrate |
Related compounds |
Copper(II) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zinc nitrate is an inorganic chemical compound with the formula Zn(NO3)2 . This white, crystalline solid is highly deliquescent and is typically encountered as a hexahydrate Zn(NO3)2•6H2O. It is soluble in both water and alcohol.
Synthesis and reactions
Zinc nitrate is usually prepared by dissolving zinc in nitric acid, this reaction is concentration dependent, with a reaction in concentrated acid also forming ammonium nitrate:
- Zn + 2 HNO3 (diluted) → Zn(NO3)2 + H2
- 4 Zn + 10 HNO3 (concentrated) → 4 Zn(NO3)2 + NH4NO3 + 3 H2O
On heating, it undergoes thermal decomposition to form zinc oxide, nitrogen dioxide and oxygen.
- 2 Zn(NO3)2 → 2 ZnO + 4 NO2 + O2
Applications
Zinc nitrate has no large scale application but is used on a laboratory scale for the synthesis of coordination polymers,[1] its controlled decomposition to zinc oxide has also been used for the generation of various ZnO based structures, including nanowires.[2]
It can be used as a mordant in dyeing. An example reaction gives a precipitate of zinc carbonate:
References
- ↑ Barnett, Sarah A; Champness, Neil R (November 2003). "Structural diversity of building-blocks in coordination framework synthesis—combining M(NO3)2 junctions and bipyridyl ligands". Coordination Chemistry Reviews. 246 (1–2): 145–168. doi:10.1016/S0010-8545(03)00121-8.
- ↑ Greene, Lori E.; Yuhas, Benjamin D.; Law, Matt; Zitoun, David; Yang, Peidong (September 2006). "Solution-Grown Zinc Oxide Nanowires". Inorganic Chemistry. 45 (19): 7535–7543. doi:10.1021/ic0601900.
Salts and covalent derivatives of the nitrate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HNO3 | He | ||||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
C | NO− 3, NH4NO3 |
O | FNO3 | Ne | ||||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr | ||
| RbNO3 | Sr(NO3)2 | Y | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 | ||
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |||
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||