Scandium nitrate
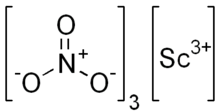 | |
| Names | |
|---|---|
| IUPAC name
scandium(3+) trinitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.033.350 |
| EC Number | 236-701-5 |
PubChem CID |
|
| |
| |
| Properties | |
| Sc(NO3)3 | |
| Molar mass | 230.97 g/mol |
| Appearance | off-white crystals |
| Solubility in other solvents | water and strong mineral acids |
| Related compounds | |
Related compounds |
Scandium(III) chloride Scandium(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Scandium(III) nitrate, Sc(NO3)3, is an ionic compound. It is an oxidizer, as all nitrates are. It is applied in optical coatings, catalysts, electronic ceramics and the laser industry.
References
Salts and covalent derivatives of the nitrate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HNO3 | He | ||||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
C | NO− 3, NH4NO3 |
O | FNO3 | Ne | ||||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr | ||
| RbNO3 | Sr(NO3)2 | Y | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 | ||
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |||
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.