Tesamorelin
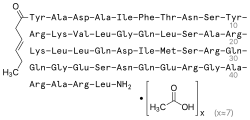 | |
| Clinical data | |
|---|---|
| Trade names | Egrifta |
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a611035 |
| Pregnancy category |
|
| Routes of administration | Subcutaneous injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ≤4%[1] |
| Metabolism | Proteolysis |
| Elimination half-life | 26–38 min |
| Excretion | Renal/proteolysis |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C221H366N72O67S |
| Molar mass | 5135.86 g/mol |
| 3D model (JSmol) | |
| |
| |
Tesamorelin (INN) (trade name Egrifta) is a synthetic form of growth-hormone-releasing hormone (GHRH) which is used in the treatment of HIV-associated lipodystrophy. It is produced and developed by Theratechnologies, Inc. of Canada. The drug is a synthetic peptide consisting of all 44 amino acids of human GHRH with the addition of a trans-3-hexenoic acid group.[2]
See also
References
- ↑ "Egrifta (tesamorelin for injection) for Subcutaneous Use. U.S. Full Prescribing Information" (PDF). EMD Serono, Inc. Retrieved 9 April 2016.
- ↑ "FDA Application Chemistry Review" (PDF).
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.