Taenia solium
| Taenia solium | |
|---|---|
| Scolex (head) of Taenia solium | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Cestoda |
| Order: | Cyclophyllidea |
| Family: | Taeniidae |
| Genus: | Taenia |
| Species: | T. solium |
| Binomial name | |
| Taenia solium | |
Taenia solium is the so-called pork tapeworm belonging to cyclophyllid cestodes in the family Taeniidae. It is an intestinal mainly zoonotic parasite found throughout the world, and is most prevalent in countries where pork is eaten, and in its more dangerous secondary form wherever faecally contaminated water is drunk, having been infected by primary, human hosts. The adult worm has as its main host humans and has a flat, ribbon-like body, which is white and measures 2 to 3 metres long or more. Its tiny long attachment, the scolex, contains duodenum wall-suckers and a rostellum as organs of attachment. The main body, the strobila, consists of a chain of segments known as proglottids. Each proglottid is little more than a self-sustainable, very lightly ingestive, reproductive unit; hence, the tapeworm is a hermaphrodite. It completes its life cycle in humans as the definitive host and often pigs as intermediate host. It may be transmitted to pigs through human feces contaminating their fodder, and back to humans wherever primary hosts through uncooked or undercooked pork bearing small cysts. Pigs ingest embryonated eggs called morula, which develop into larvae, the oncospheres, and ultimately into infective larvae, cysticerci. A cysticercus grows into an adult worm in human small intestines. Primary hosts often present no pathological symptoms. Secondary human hosts, infected by faecally contaminated water or matter by definition develop its complication cysticercosis, the most harmful and chronic form of which is neurocysticercosis. Primary hosts can be easily treated with oral medicines. Treatment of secondary hosts is more difficult but possible.
Human primary hosting is best diagnosed by microscopy of eggs in faeces, often triggered by spotting proglottids (segments). In secondary hosting, imaging techniques such as computed tomography and nuclear magnetic resonance are often employed. Blood samples can also be tested using antibody reaction of enzyme-linked immunosorbent assay.
Description
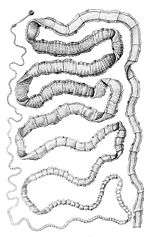

Adult T. solium is a triploblastic acoelomate, having no body cavity. It is normally 2 to 3 m in length, but can become much larger, sometimes over 8 m long. It is white in colour and flattened into a ribbon-like body.
The anterior end is a knob-like attachment organ (sometimes mistakenly-referred to as a "head") called a scolex, 1 mm in diameter. The scolex bears four radially arranged suckers (acetabula) that surround the rostellum. These are the organs of adhesive attachment to the intestinal wall of the host. The rostellum is armed with two rows of spiny hooks, which are chitinous in nature. Its 22 to 32 rostellar hooks can be differentiated into short (130-µm) and long (180-µm) types. After a short neck is the elongated body, the strobila. The entire body is covered by a covering called tegument, which is an absorptive layer consisting of a mat of minute hair-like microtriches. The strobila is divided into segments called proglottids, 800 to 900 in number. Body growth starts from the neck region, so the oldest proglottids are at the posterior end. Thus, the three distinct proglottids are immature proglottids towards the neck, mature proglottids in the middle, and gravid proglottids at the posterior end. A monoecious species, each mature proglottid contains a set of male and female reproductive systems. The numerous testes and a bilobed ovary open into a common genital pore. The oldest gravid proglottids are full of fertilised eggs,[1][2][3][4]
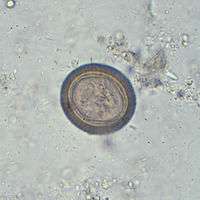
If released early enough in the digestive tract and not passed, fertilised eggs can mature using upper tract digestive enyzmes and the tiny larvae migrate to form cysticerci in humans. These have three morphologically distinct types.[5] The common one is the ordinary "cellulose" cysticercus, which has a fluid-filled bladder 0.5 to 1.5 cm in length and an invaginated scolex. The intermediate form has a scolex. The "racemose" has no evident scolex, but is believed to be larger. They can be 20 cm in length and have 60 ml of fluid, and 13% of patients with neurocysticercosis can have all three types in the brain.
Life cycle
The life cycle of T. solium is indirect. It passes through pigs or other animals, as intermediate hosts, into humans, as definitive hosts. In humans the infection can be relatively short or long lasting, and in the latter case if reaching the brain can last for life. From humans, the eggs are released in the environment where they await ingestion by another host. In the secondary host, the eggs develop into oncospheres which bore through the intestinal wall and migrate to other parts of the body where the cysticerci form. The cycticerci can survive for several years in the animal.[6]
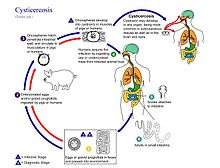
Definitive host
Humans are colonized by the larval stage, the cysticercus, from undercooked 'measly' pork or other meat. Each microscopic cysticercus is oval in shape, containing an inverted scolex (specifically "protoscolex"), which everts once the organism is inside the small intestine. This process of evagination is stimulated by bile juice and digestive enzymes (of the host). Using the scolex, it anchors to the intestinal wall. It grows in size using nutrients from the surroundings. Its strobila lengthens as new proglottids are formed at the foot of the neck. In 10–12 weeks after initial colonization, it is an adult worm. As a hermaphrodite, it reproduces by self-fertilisation, or cross-fertilisation if gametes are exchanged between two different proglottids. Spermatozoa fuse with the ova in the fertilisation duct, where the zygotes are produced. The zygote undergoes holoblastic and unequal cleavage resulting in three cell types, small, medium and large (micromeres, mesomeres, megameres). Megameres develop into a syncytial layer, the outer embryonic membrane; mesomeres into the radially striated inner embryonic membrane or embryophore; micromeres become the morula. The morula transforms into a six-hooked embryo known as an oncosphere, or hexacanth ("six hooked") larva. A gravid proglottid can contain more than 50,000 embryonated eggs. Gravid proglottids often rupture in the intestine, liberating the oncospheres in faeces. Intact gravid proglottids are shed off in groups of four or five. The free eggs and detached proglottids are spread through the host's defecation (peristalsis). Oncospheres can survive in the environment for up to two months.[2][7]
Intermediate host
Pigs are the most common host who ingest such eggs in traces of human faeces, mainly from vegetation contaminated with it such as from water bearing traces of it. The embryonated eggs enter intestine where they hatch into motile oncospheres. The embryonic and basement membranes are removed by the host's digestive enzymes (particularly pepsin). Then the free oncospheres attach on the intestinal wall using their hooks. With the help of digestive enzymes from the penetration glands, they penetrate the intestinal mucosa to enter blood and lymphatic vessels. They move along the general circulatory system to various organs, and large numbers are cleared in the liver. The surviving oncospheres preferentially migrate to striated muscles, as well as the brain, liver, and other tissues, where they settle to form cysts — cysticerci. A single cysticercus is spherical, measuring 1–2 cm in diameter, and contains an invaginated protoscolex. The central space is filled with fluid like a bladder, hence it is also called bladder worm. Cysticerci are usually formed within 70 days and may continue to grow for a year.[8]
Humans are also accidental secondary hosts when they are colonized by embryonated eggs, either by auto-colonization or ingestion of contaminated food. As in pigs, the oncospheres hatch and enter blood circulation. When they settle to form cysts, clinical symptoms of cysticercosis appear. The cysticercus is often called the metacestode.
Epidemiology
T. solium is found worldwide, but its two distinctive forms rely on eating unaudited, undercooked pork or on ingesting faecally contaminated water or food (respectively). These invectives vary by location, particularly cooking, meat-checking, water and hygiene education standards. Because pig meat is the intermediate source of the intestinal parasite, rotation of the full life cycle occurs in regions where humans live in close contact with pigs and eat undercooked pork. However humans can also act as secondary hosts, which is a more pathological, harmful stage triggered by oral contamination. High prevalences are reported among many places with poorer than average water hygiene or even mildly contaminated water especially with a pork-eating heritage such as Mexico, Latin America, West Africa, Russia, India, Pakistan, Manchuria, and Southeast Asia.[9] In Europe it remains most endemic in pockets of Slavic countries and among global travellers taking inadequate precautions in eating pork especially.[3][10]
The secondary host form, human cysticercosis, predominates in areas where poor hygiene allows for mild faecal contamination of food, soil, or water supplies. Prevalence rates in the United States have shown immigrants from Mexico, Central and South America, and Southeast Asia bear the brunt of cases of cysticercosis caused by the ingestion of microscopic, long-lasting and hardy tapeworm eggs.[11] Taeniasis and cysticercosis are rare in large Muslim and Jewish heterogenous communities with safe water, as the religions forbid consumption of pork. The intra-bodily cysts condition can infect affect wide areas with faecally contaminated water sources or food once infected by a tapeworm-bearing visitor such as in or downstream of cosmopolitan cities with poor sewage and water treatment. It can occur in populations that neither eat pork nor share environments with pigs. A review of T. solium cysticercosis in West Africa concluded that its prevalence was not affected by any religion in particular.[12]
For example in 1990 and 1991 four unrelated members of an Orthodox Jewish community in New York City developed recurrent seizures and brain lesions, which were found to have been caused by T. solium. All had housekeepers from Latin American countries who were suspected to be source of the infections or others handling their food.[13][14]
See also
References
- ↑ Pawlowski, Z.S.; Prabhakar, Sudesh (2002). "Taenia solium: basic biology and transmission". In Gagandeep Singh, Sudesh Prabhakar. Taenia solium Cysticercosis from Basic to Clinical Science. Wallingford, Oxon, UK: CABI Pub. pp. 1–14. ISBN 9780851998398.
- 1 2 Carter, Burton J. Bogitsh, Clint E. (2013). Human Parasitology (4th ed.). Amsterdam: Academic Press. pp. 241–244. ISBN 9780124159150.
- 1 2 Gutierrez, Yezid (2000). Diagnostic Pathology of Parasitic Infections with Clinical Correlations (2nd ed.). New York [u.a.]: Oxford University Press. pp. 635–652. ISBN 9780195121438.
- ↑ Willms, Kaethe (2008). "Morphology and Biochemistry of the Pork Tapeworm, Taenia solium". Current Topics in Medicinal Chemistry. 8 (5): 375–382. doi:10.2174/156802608783790875. PMID 18393900.
- ↑ Rabiela, MT; Rivas, A; Flisser, A (November 1989). "Morphological types of Taenia solium cysticerci". Parasitology Today. 5 (11): 357–359. doi:10.1016/0169-4758(89)90111-7. PMID 15463154.
- ↑ Biology. (2013, January 10). Retrieved from https://www.cdc.gov/parasites/taeniasis/biology.html
- ↑ Mayta, Holger (2009). Cloning and Characterization of Two Novel Taenia Solium Antigenic Proteins and Applicability to the Diagnosis and Control of Taeniasis/cysticercosis. ProQuest. pp. 4–12. ISBN 9780549938996.
- ↑ Garcia, Oscar H. Del Brutto, Hector H. (2014). "Taenia solium: Biological Characteristics and Life Cycle". Cysticercosis of the Human Nervous System (1., 2014 ed.). Berlin: Springer-Verlag Berlin and Heidelberg GmbH & Co. KG. pp. 11–21. ISBN 978-3-642-39021-0.
- ↑ Reeder, P.E.S. Palmer, M.M. (2001). Imaging of Tropical Diseases : With Epidemiological, Pathological, and Clinical Correlation (2 (revised) ed.). Heidelberg, Germany: Springer-Verlag. pp. 641–642. ISBN 978-3-540-56028-9.
- ↑ Hansen, NJ; Hagelskjaer, LH; Christensen, T (1992). "Neurocysticercosis: a short review and presentation of a Scandinavian case". Scandinavian Journal of Infectious Diseases. 24 (3): 255–62. doi:10.3109/00365549209061330. PMID 1509231.
- ↑ Flisser A. (May 1988). "Neurocysticercosis in Mexico". Parasitology Today. 4 (5): 131–137. doi:10.1016/0169-4758(88)90187-1. PMID 15463066.
- ↑ Melki, Jihen; Koffi, Eugène; Boka, Marcel; Touré, André; Soumahoro, Man-Koumba; Jambou, Ronan (2018). "Taenia solium cysticercosis in West Africa: status update". Parasite. 25: 49. doi:10.1051/parasite/2018048. ISSN 1776-1042. PMID 30230445.

- ↑ Dworkin, Mark S. (2010). Outbreak Investigations Around the World: Case Studies in Infectious Disease. Jones and Bartlett Publishers. pp. 192–196. ISBN 978-0-7637-5143-2. Retrieved August 9, 2011.
- ↑ Schantz; Moore, Anne C.; et al. (September 3, 1992). "Neurocysticercosis in an Orthodox Jewish Community in New York City". New England Journal of Medicine. 327 (10): 692–695. doi:10.1056/NEJM199209033271004.