Sodium orthovanadate
 | |
| Names | |
|---|---|
| IUPAC name
Sodium vanadate(V) | |
| Other names
Sodium vanadium oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ECHA InfoCard | 100.033.883 |
PubChem CID |
|
| RTECS number | YW1120000 |
| |
| |
| Properties | |
| Na3VO4 | |
| Molar mass | 183.908 g/mol |
| Appearance | white powder |
| Density | 2.16 g/cm3, solid |
| Melting point | 858 °C (1,576 °F; 1,131 K) |
| 22.17 g/100 mL | |
| Solubility | insoluble in ethanol |
| Structure | |
| cubic | |
| Thermochemistry | |
Heat capacity (C) |
164.8 J/mol K |
Std molar entropy (S |
190 J/mol K |
Std enthalpy of formation (ΔfH |
−1757 kJ/mol |
| Hazards | |
| Main hazards | Harmful. |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
330 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium orthovanadate is the inorganic compound with the chemical formula Na3VO4·2H2O (sodium orthovanadate dihydrate). It is a salt of the VO3−
4 oxyanion. It is a colorless, water-soluble solid.[1]
Synthesis and structure
Sodium orthovanadate is produced by dissolving vanadium(V) oxide in a solution of sodium hydroxide:
- V2O5 + 6 NaOH → 2 Na3VO4 + 3 H2O
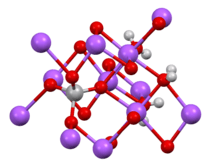
The salt features tetrahedral VO3−
4 centers linked to octahedral Na+ sites.[2]
Reactions
Acidification of orthovanadate induces condensation to polyoxovanadates, specifically decavanadate.[3]
Vanadates exhibit a variety of biological activities, in part because they serve as structural mimics of phosphates.[4][5] It acts as a competitive inhibitor of ATPases, alkaline and acid phosphatases, and protein-phosphotyrosine phosphatases,[6] and its inhibitory effects can be reversed by dilution or the addition of Ethylenediaminetetraacetic acid (EDTA).[7]
Orthovanadate is activated by boiling and adjusting pH to ~10; this depolymerizes decavanadate into the active inhibitor, monovanadate.[6]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Kato, K.; Takayama-Muromachi, E. (1987). "Die Struktur des Trinatriumvanadattrihydrats" [The structure of trisodium vanadate trihydrate]. Acta Crystallogr. C43: 1030–1032. doi:10.1107/S0108270187093120.
- ↑ Klemperer, W. G.; Yaghi, O. (1983). "Tetrabutylammonium Trihydrogen Decavanadate(V)". Inorg. Synth. 27: 83. doi:10.1002/9780470132586.ch15.
- ↑ Korbecki, Jan; Baranowska-Bosiacka, Irena; Gutowska, Izabela; Chlubek, Dariusz (2012). "Biochemical and medical importance of vanadium compounds" (PDF). Acta Biochim. Polon. 59: 195–200.
- ↑ Crans, D. C.; Chatterjee, P. B. (2013). "Vanadium biochemistry". In Reedijk, Jan; Poeppelmeier, Kenneth. Comprehensive Inorganic Chemistry II. 3. pp. 323–342. doi:10.1016/B978-0-08-097774-4.00324-7. ISBN 978-0-08-097774-4.
- 1 2 "Sodium orthovanadate" (PDF). Sigma-Aldrich. Retrieved September 7, 2018.
- ↑ Biolabs, New England. "Sodium Orthovanadate (Vanadate) | NEB". www.neb.com. Retrieved 2018-09-07.
