Red Queen hypothesis
The Red Queen hypothesis, also referred to as Red Queen's, Red Queen's race or the Red Queen effect, is an evolutionary hypothesis which proposes that organisms must constantly adapt, evolve, and proliferate not merely to gain reproductive advantage, but also simply to survive while pitted against ever-evolving opposing organisms in a constantly changing environment. The hypothesis intends to explain two different phenomena: the constant extinction rates as observed in the paleontological record caused by co-evolution between competing species,[1] and the advantage of sexual reproduction (as opposed to asexual reproduction) at the level of individuals.[2]
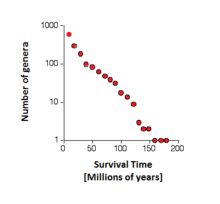
Leigh Van Valen proposed the hypothesis to explain the "Law of Extinction",[1] showing that, in many populations, the probability of extinction does not depend on the lifetime of the population, instead being constant over millions of years for a given population. This could be explained by the coevolution of species, where established species have evolved cooperatively by assuming adaptive coevolutionary dependencies. These complementary relationships develop through graduated symbiosis, directing punctuated advantages specialized enough to ensure a greater survivability and fitness rate for both species.[3] Indeed, an adaptation in a population of one species (e.g. predators, parasites) may change the natural selection pressure on a population of another species (e.g. prey, hosts), giving rise to common antagonistic coevolutions. If this positive feedback occurs reciprocally, a potential dynamic coevolution may result.[4]
In another idea, the Red Queen hypothesis is used independently by Hartung[5] and Bell to explain the evolution of sex,[2] by John Jaenike to explain the maintenance of sex[6] and W. D. Hamilton to explain the role of sex in response to parasites.[7][8] In all cases, sexual reproduction confers species variability and a faster generational response to selection by making offspring genetically unique. Sexual species are able to improve their genotype in changing conditions. Consequently, co-evolutionary interactions, between host and parasite, for example, may select for sexual reproduction in hosts in order to reduce the risk of infection. Oscillations in genotype frequencies are observed between parasites and hosts in an antagonistic coevolutionary way[9] without necessitating changes to the phenotype. In multi-host and multi-parasite coevolution, the Red Queen dynamics could affect what host and parasite types will become dominant or rare.[10]
Etymology
The phenomenon's name is derived from a statement that the Red Queen made to Alice in Lewis Carroll's Through the Looking-Glass in her explanation of the nature of Looking-Glass Land:
Now, here, you see, it takes all the running you can do, to keep in the same place.[11]
Van Valen coined the hypothesis "Red Queen" because under this interpretation, species have to "run" or evolve in order to stay in the same place, or else go extinct.
Leigh Van Valen (1973) proposed the metaphor of an evolutionary arms race, which was appropriate for the description of biological processes with dynamics similar to arms races. In respective processes, an adaptation in a population of one species (e.g., predators, parasites) may change the selection pressure on a population of another species (e.g., prey, host), giving rise to an antagonistic coevolution.[4]
Interspecies arms race:
- A number of predator/prey couple where the weapon involved is the running speed.
"The rabbit runs faster than the fox, because the rabbit is running for his life while the fox is only running for his dinner." Aesop[12]
- The interactions between parasitoid wasps and insect larvae, necessary for the parasitic wasp's life cycle, are also a good illustration of an arms race. Indeed, some evolutionary strategy was found by both partners to respond to the pressure generated by the mutual association of lineages. For example, the parasitoid wasp group, Campoletis sonorensis, is able to fight against the immune system of its hosts, Heliothis virescens (Lepidopteran) with the association of a polydnavirus (PDV) (Campoletis sonorensis PDV). During the oviposition process, the parasitoid transmits the virus (CsPDV) to the insect larva. The CsPDV will alter the physiology, growth and development of the infected insect larvae to the benefit of the parasitoid.[13]
The emergence of the concept and illustrations of it
Van Valen proposed the Red Queen hypothesis as an explanatory tangent to his proposed "Law of Extinction" (also 1973), which he derived from observation of constant probabilities of extinction within families of organisms across geological time. Put differently, Van Valen found that the ability of a family of organisms to survive does not improve over time, and that the lack of correlation between age and extinction is suggestive of a random process.[1] The Red Queen hypothesis as formulated by Van Valen provides a conceptual underpinning to discussions of evolutionary arms races, even though a direct test of the hypothesis remains elusive, particularly at the macroevolutionary level. This concept remains similar to that of a system obeying a self-organized criticality.[14]
For example, because every improvement in one species will lead to a selective advantage for that species, variation will normally continuously lead to increases in fitness in one species or another. However, since different species tend to co-evolve, an improvement in one species implies that it will get a competitive advantage over the other species, and thus be able to capture a larger share of the resources available to all. This means that the fitness increase in one evolutionary system will tend to lead to the fitness decrease in another system. The only way that a species involved in a competition for resources can maintain its fitness relative to other competing species is by improving its specific fitness. (From Heylighen, 2000)
The most obvious example of this effect are the "arms races" between predators and prey (e.g., Vermeij, 1987), where the only way predators can compensate for a better defense by the prey (e.g., rabbits running faster than their parents) is by developing a better offense (e.g.. foxes running faster than their parents). In this case, we might consider the relative improvements (rabbits running faster than foxes or vice versa) to be also absolute improvements in fitness. (From Heylighen, 2000)
Discussions of sex and reproduction were not part of Van Valen's Red Queen hypothesis, which addressed evolution at scales above the species level. The microevolutionary version of the Red Queen hypothesis was proposed by Bell (1982), also citing Lewis Carroll, but not citing Van Valen .
Application to human conflict
One can apply such arms races to human conflict and interpret them as a prominent cause of conflict. According to Azar Gat, the Red Queen effect arises when two competing groups find themselves in a security dilemma. The security dilemma results when a group takes defensive measures (which possess inherent offensive capabilities) to improve their security, triggering a military arms race. This arms race, much like the example previously referenced, causes each side to consume ever increasing amounts of resources in order to outpace the other and to gain an advantage. If an advantage is gained, the arms race is over and the group with more resources has won. However, typically both sides continue to match each other stride for stride, thus triggering the Red Queen effect, as no matter how many resources each side invests, neither is able to gain an advantage. The situation somewhat resembles the prisoner's dilemma. Neither side can stop the arms race, due to mutual suspicion and fears that the other group will gain a significant tactical advantage. Because of this, the Red Queen effect is a common outcome of inter-human competition and conflict.[15]
The paradox of sex: The "cost" of males
Science writer Matt Ridley popularized the term in connection with sexual selection in his 1993 book The Red Queen, in which he discussed the debate in theoretical biology over the adaptive benefit of sexual reproduction to those species in which it appears. The connection of the Red Queen to this debate arises from the fact that the traditionally accepted Vicar of Bray hypothesis only showed adaptive benefit at the level of the species or group, not at the level of the gene (although the protean "Vicar of Bray" adaptation is very useful to some species that belong to the lower levels of the food chain). By contrast, a Red-Queen-type thesis that organisms are running cyclic arms races with their parasites can explain the utility of sexual reproduction at the level of the gene by positing that the role of sex is to preserve genes that are currently disadvantageous, but that will become advantageous against the background of a likely future population of parasites.
Further evidence was observed of the Red Queen to see allelic effects under sexual selection. The Red Queen Hypothesis leads to the understanding that allelic recombination is advantageous for populations that engage in aggressive biotic interactions, such as predator-prey or parasite-host interactions. In cases of parasite-host relations, sexual reproduction can quicken the production of new multi-locus genotypes allowing the host to escape parasites that have adapted to the prior generations of typical hosts.[16] Mutational effects can be represented by models to describe how recombination through sexual reproduction can be advantageous. According to the mutational deterministic hypothesis, if the deleterious mutation rate is high, and if those mutations interact to cause a general decline in organismal fitness, then sexual reproduction provides an advantage over asexually reproducing organisms by allowing populations to eliminate the deleterious mutations not only more rapidly, but also most effectively.[16] Recombination is one of the fundamental means that explain why many organisms have evolved to reproduce sexually.
Sexual organisms must spend resources to find mates. In the case of sexual dimorphism, usually one of the sexes contributes more to the survival of their offspring (usually the mother). In such cases, the only adaptive benefit of having a second sex is the possibility of sexual selection, by which organisms can improve their genotype.
Observational evidence
Evidence for this explanation for the evolution of sex is provided by the comparison of the rate of molecular evolution of genes for kinases and immunoglobulins in the immune system with genes coding other proteins. The genes coding for immune system proteins evolve considerably faster.[17][18]
Further evidence for the Red Queen hypothesis was provided by observing long‐term dynamics and parasite coevolution in a mixed sexual and asexual population of snails (Potamopyrgus antipodarum). The number of sexuals, the number of asexuals, and the rates of parasite infection for both were monitored. It was found that clones that were plentiful at the beginning of the study became more susceptible to parasites over time. As parasite infections increased, the once-plentiful clones dwindled dramatically in number. Some clonal types disappeared entirely. Meanwhile, sexual snail populations remained much more stable over time.[19][20]
In 2011, researchers used the microscopic roundworm Caenorhabditis elegans as a host and the pathogenic bacterium Serratia marcescens to generate a host–parasite coevolutionary system in a controlled environment, allowing them to conduct more than 70 evolution experiments testing the Red Queen hypothesis. They genetically manipulated the mating system of C. elegans, causing populations to mate either sexually, by self-fertilization, or a mixture of both within the same population. Then they exposed those populations to the S. marcescens parasite. It was found that the self-fertilizing populations of C. elegans were rapidly driven extinct by the coevolving parasites, while sex allowed populations to keep pace with their parasites, a result consistent with the Red Queen hypothesis.[21][22]
Currently, there is no consensus among biologists on the main selective forces maintaining sex. The competing models to explain the adaptive function of sex have been reviewed by Birdsell and Wills.[23]
Historical studies
The influence of heterogeneity in species genomes has been recognized and studied since the time of Darwin (1876) in the areas of heterosis (hybrid vigor), inbreeding and genetic deterioration, operating on the theory that lessening of the choice of gene variants and of potential cooperation among different gene types limits the capabilities of the restricted organism.[24] Resultant genetic drift or genetic isolates reduce available corresponding genetic material volumes, where variable limitations can catalyze a consequential population bottleneck.
A study published in 2013 in Science which examined the history of groups of extinct mammals illustrates the failure to adapt and evolve new species when confronted by a deteriorating environment.[25][26] An interesting insight can be obtained from physical research.[27]
Evolution of aging
The Red Queen hypothesis has been also invoked by some authors to explain evolution of aging.[28][29] The main idea is that aging is favored by natural selection since it allows faster adaptation to changing conditions, especially in order to keep pace with the evolution of pathogens, predators and prey.[29]
Court jester hypothesis
A competing evolutionary idea is the court jester hypothesis, which indicates that an arms race is not the driving force of evolution on a large scale, but rather it is abiotic factors.[30][31]
See also
References
- 1 2 3 Van Valen, Leigh (1973). "A new evolutionary law" (PDF). Evolutionary Theory. 1: 1–30.
- 1 2 Bell, G. (1982). The Masterpiece Of Nature: The Evolution and Genetics of Sexuality. University of California Press, Berkeley, 378 pp.
- ↑ Magain, N; Sérusiaux, E (2014). "Do photobiont switch and cephalodia emancipation act as evolutionary drivers in the lichen symbiosis? A case study in the Pannariaceae (Peltigerales)". PLoS ONE. 9: e89876. Bibcode:2014PLoSO...989876M. doi:10.1371/journal.pone.0089876. PMC 3933699. PMID 24587091.
- 1 2 Dawkins, Richard; Krebs, John R. (1979). "Arms races between and within species". Proceedings of the Royal Society of London B. 205 (1161): 489–511. Bibcode:1979RSPSB.205..489D. doi:10.1098/rspb.1979.0081. PMID 42057.
- ↑ . Genome Parliaments and Sex with the Red Queen. In: Alexander, R.D., Tinkle, D. W., Eds. Natural Selection and Social Behavior: Recent Research and New Theory. New York: Chiron Press, 1981, 382-402
- ↑ Jaenike, J. (1978). "An hypothesis to account for the maintenance of sex within populations". Evolutionary Theory. 3: 191–194.
- ↑ Hamilton, W. D.; Axelrod, R.; Tanese, R. (1990). "Sexual reproduction as an adaptation to resist parasites". Proceedings of the National Academy of Sciences of the USA. 87: 3566–3573. Bibcode:1990PNAS...87.3566H. doi:10.1073/pnas.87.9.3566. PMC 53943. PMID 2185476.
- ↑ Hamilton, W. D. (1980). "Sex versus non-sex versus parasite". Oikos. 35: 282–290. doi:10.2307/3544435.
- ↑ Rabajante, J.; et al. (2015). "Red Queen dynamics in multi-host and multi-parasite interaction system". Scientific Reports. 5: 10004. Bibcode:2015NatSR...510004R. doi:10.1038/srep10004. ISSN 2045-2322. PMC 4405699. PMID 25899168.
- ↑ Rabajante, J; et al. (2016). "Host-parasite Red Queen dynamics with phase-locked rare genotypes". Science Advances. 2: e1501548. Bibcode:2016SciA....2E1548R. doi:10.1126/sciadv.1501548. ISSN 2375-2548.
- ↑ Carroll, Lewis (1991) [1871]. "2: The Garden of Live Flowers". Through the Looking-Glass (The Millennium Fulcrum Edition 1.7 ed.). Project Gutenberg. Retrieved 26 September 2017.
- ↑ Dawkins, Richard. The Selfish Gene:30th Anniversary Edition. Oxford University Press, 2006, p. 250.
- ↑ Kent, Shelby; Bruce, Webb (1999). "Polydnavirus-mediated suppression of insect immunity". Journal of Insect Physiology. 45 (5): 507–514. doi:10.1016/S0022-1910(98)00144-9].
- ↑ "Self-organized criticality in living systems". Physics Letters A. 203: 29–32. 1995. arXiv:adap-org/9401001. Bibcode:1995PhLA..203...29A. doi:10.1016/0375-9601(95)00372-A.
- ↑ Gat, Azar (2006). War in Human Civilization. New York: Oxford University Press. pp. 98–99. ISBN 0-19-926213-6.
- 1 2 Cooper, T. F.; Lenski, R. E.; Elena, S. F. (2005). "Parasites and mutational load: An experimental test of a pluralistic theory for the evolution of sex". Proceedings of the Royal Society B: Biological Sciences. Royal Society. 272: 311–317. doi:10.1098/rspb.2004.2975. PMC 1634976. PMID 15705557.
- ↑ Kuma, K.; Iwabe, N.; Miyata, T. (1995). "Functional constraints against variations on molecules from the tissue-level: Slowly evolving brain-specific genes demonstrated by protein-kinase and immunoglobulin supergene families". Molecular Biology and Evolution. 12 (1): 123–130. doi:10.1093/oxfordjournals.molbev.a040181. PMID 7877487.
- ↑ Wolfe, K. H.; Sharp, P. M. (1993). "Mammalian gene evolution: Nucleotide-sequence divergence between mouse and rat". Journal of Molecular Evolution. 37 (4): 441–456. Bibcode:1993JMolE..37..441W. doi:10.1007/BF00178874. PMID 8308912.
- ↑ Jokela, Jukka; Dybdahl, Mark; Lively, Curtis (2009). "The Maintenance of Sex, Clonal Dynamics, and Host-Parasite Coevolution in a Mixed Population of Sexual and Asexual Snails". The American Naturalist. 174 (s1): S43–S53. doi:10.1086/599080. PMID 19441961.
- ↑ "Parasites May Have Had Role in Evolution of Sex". Science Daily. 31 July 2009. Retrieved 19 September 2011.
- ↑ Morran, Levi T.; Schmidt, Olivia G.; Gelarden, Ian A., II; Parrish, Raymond C.; Lively, Curtis M. (2011). "Running with the Red Queen: Host-Parasite Coevolution Selects for Biparental Sex". Science. 333 (6039): 216–218. Bibcode:2011Sci...333..216M. doi:10.1126/science.1206360. PMC 3402160. PMID 21737739.
- ↑ "Sex — as We Know It — Works Thanks to Ever-Evolving Host-Parasite Relationships, Biologists Find". Science Daily. 9 July 2011. Retrieved 19 September 2011.
- ↑ Birdsell JA, Wills C (2003). The evolutionary origin and maintenance of sexual recombination: A review of contemporary models. Evolutionary Biology Series >> Evolutionary Biology, Vol. 33 pp. 27-137. MacIntyre, Ross J.; Clegg, Michael, T (Eds.), Springer. Hardcover ISBN 978-0306472619, ISBN 0306472619 Softcover ISBN 978-1-4419-3385-0.
- ↑ Birchler, James A.; Yao, Hong; Chudalayandi, Sivanandan (August 2006). "Unraveling the genetic basis of hybrid vigor". Proceedings of the National Academy of Sciences. 103 (35): 12957–12958. Bibcode:2006PNAS..10312957B. doi:10.1073/pnas.0605627103. PMC 1559732. PMID 16938847.
- ↑ Quental, Tiago B.; Marshall, Charles R. (June 20, 2013). "How the Red Queen Drives Terrestrial Mammals to Extinction". Science Express. 341: 290–292. Bibcode:2013Sci...341..290Q. doi:10.1126/science.1239431. Retrieved June 26, 2013.
- ↑ Sanders, Robert (June 20, 2013). "The Red Queen was right: Life must continually evolve to avoid extinction". University of California Berkeley Media Relations. Retrieved June 26, 2013.
- ↑ "Physics and Math Shed New Light on Biology by Mapping the Landscape of Evolution" (News release). American Institute of Physics (AIP). 8 August 2012
- ↑ Mitteldorf, Josh; Pepper, John (2009). "Senescence as an adaptation to limit the spread of disease". Journal of Theoretical Biology. 260 (2): 186–195. doi:10.1016/j.jtbi.2009.05.013. ISSN 0022-5193.
- 1 2 Lenart, Peter; Bienertová-Vašků, Julie (2016). "Keeping up with the Red Queen: the pace of aging as an adaptation". Biogerontology. doi:10.1007/s10522-016-9674-4. ISSN 1389-5729.
- ↑ Benton, Michael J. (2009). "The Red Queen and the Court Jester: Species Diversity and the Role of Biotic and Abiotic Factors Through Time". Science. 323 (5915): 728–732. Bibcode:2009Sci...323..728B. doi:10.1126/science.1157719. PMID 19197051.
- ↑ Sahney, S.; Benton, M.J.; Ferry, P.A. (2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land" (PDF). Biology Letters. 6 (4): 544–547. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
Further reading
- Francis Heylighen (2000): "The Red Queen Principle", in: F. Heylighen, C. Joslyn and V. Turchin (editors): Principia Cybernetica Web (Principia Cybernetica, Brussels), URL: http://pespmc1.vub.ac.be/REDQUEEN.html.
- Pearson, Paul N. (2001) Red Queen hypothesis Encyclopedia of Life Sciences http://www.els.net
- Ridley, M. (1995) The Red Queen: Sex and the Evolution of Human Nature, Penguin Books, ISBN 0-14-024548-0
- Vermeij, G.J. (1987). Evolution and escalation: An ecological history of life. Princeton University Press, Princeton, NJ.