PI3K/AKT/mTOR pathway
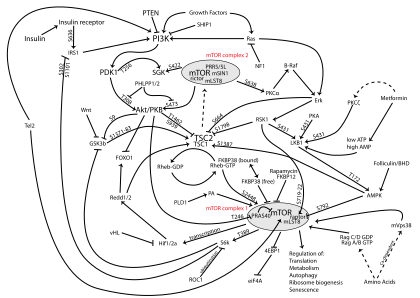
The PI3K/AKT/mTOR pathway is an intracellular signaling pathway important in regulating the cell cycle. Therefore, it is directly related to cellular quiescence, proliferation, cancer, and longevity. PI3K activation phosphorylates and activates AKT, localizing it in the plasma membrane.[1] AKT can have a number of downstream effects such as activating CREB,[2] inhibiting p27,[3] localizing FOXO in the cytoplasm,[3] activating PtdIns-3ps,[4] and activating mTOR[3] which can affect transcription of p70 or 4EBP1.[3] There are many known factors that enhance the PI3K/AKT pathway including EGF,[5] shh,[2] IGF-1,[2] insulin,[3] and CaM.[4] The pathway is antagonized by various factors including PTEN,[6] GSK3B,[2] and HB9.[5] In many cancers, this pathway is overactive, thus reducing apoptosis and allowing proliferation. This pathway is necessary, however, to promote growth and proliferation over differentiation of adult stem cells, neural stem cells specifically.[2] It is the difficulty in finding an appropriate amount of proliferation versus differentiation that researchers are trying to determine in order to utilize this balance in the development of various therapies.[2] Additionally, this pathway has been found to be a necessary component in neural long term potentiation.[4][7]
Proliferation of neural stem cells
Response to glucose
Neural stem cells (NSCs) in the brain must find a balance between maintaining their multipotency by self renewing and proliferating as opposed to differentiating and becoming quiescent. The PI3K/AKT pathway is crucial in this decision making process. NSCs are able to sense and respond to changes in the brain or throughout the organism. When glucose levels are high, insulin and therefore IGF is produced.[3] This signaling activates the PI3K/AKT pathway which works to promote proliferation. In this way, when there is high glucose and abundant energy in the organism, the PI3K/AKT pathway is activated and NSCs tend to proliferate. When there are low amounts of available energy, the PI3K/AKT pathway is less active and cells adopt a quiescent state. This occurs, in part, when AKT phosphorylates FOXO, keeping FOXO in the cytoplasm.[3] FOXO, when dephosphorylated, can enter the nucleus and work as a transcription factor to promote the expression of various tumor suppressors such as p27 and p21.[3] These tumor suppressors push the NSC to enter quiescence. FOXO knockouts lose the ability for cells to enter a quiescent state as well as cells losing their neural stem cell character, possibly entering a cancer like state.[3]
PTEN
The PI3K/AKT pathway has a natural inhibitor called PTEN whose function is to limit proliferation in cells, helping to prevent cancer. Knocking out PTEN has been shown to increase the mass of the brain because of the unregulated proliferation that occurs.[3] PTEN works by dephosphorylating PIP3 to PIP2 which limits AKTs ability to bind to the membrane, decreasing its activity. PTEN deficiencies can be compensated downstream to rescue differentiation or quiescence. Knocking out PTEN is not as serious as knocking out FOXO for this reason.[3]
CREB
The cAMP response element CREB is closely related to the cell decision to proliferate or not. Cells that are forced to overexpress AKT increase the amount of CREB and proliferation compared to wild type cells. These cells also express less glial and neural cell markers such as GFAP or β-tubulin.[2] This is because CREB is a transcription factor that influences the transcription of cyclin A which promotes proliferation.[2] For example, adult hippocampal neural progenitor cells need abeyance as stem cells to differentiate later. This is regulated by Shh. Shh works through a slow protein synthesis dependence, which stimulates other cascades that work synergistically with the PI3K/AKT pathway to induce proliferation. Then, the other pathway can be turned off and the effects of the PI3K/AKT pathway become insufficient in stopping differentiation.[2] The specifics of this pathway are unknown.
Roles in cancer
In Ovarian Cancer
PI3K/ AKT/mTOR pathway is a central regulator of ovarian cancer. PIM kinases are over expressed in many types of cancers and they also contribute to the regulation of ovarian cancer. PIM are directly and indirectly found to activate mTOR and its upstream effectors like AKT. Besides, PIM kinases can cause phosphorylation of IRS, which can alter PI3K. This indicates the close interaction of PIM with PI3K/ AKT/mTOR cascade and its components. Similarly, AKT has also been reported to perform the BAD phosphorylation in OC cells. PIM and the PI3K/AKT/mTOR network both can inhibit the P21 and P27 expressions in OC cells. These data suggest a strong possibility of interaction and relevance of PIM kinases and the PI3K/AKT/mTOR network in the regulation of ovarian cancer.[8]
In Breast Cancer
In many kinds of breast cancer, aberrations in the PI3K/AKT/mTOR pathway are the most common genomic abnormalities. The most common known aberrations include the PIK3CA gene mutation and the loss-of-function mutations or epigenetic silencing of phosphatase and tensin homologue (PTEN).[9] The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway is activated in approximately 30–40% of BC cases. In TNBC, oncogenic activation of the PI3K/AKT/mTOR pathway can happen as a function of overexpression of upstream regulators like EGFR, activating mutations of PIK3CA, loss of function or expression of phosphatase and tensin homolog (PTEN), and the proline-rich inositol polyphosphatase, which are downregulators of PI3K.[10] It is consistent with the hypothesis that PI3K inhibitors can overcome resistance to endocrine therapy when it is acquired
In Urothelial Cancer
PIK3CA frequently have gain of function mutations in urothelial cancer.[11] Similar to PI3Ka, PI3Kb is expressed in many different cells, and it is mainly involved in the activation of platelets and development of thrombotic diseases. Studies have shown that PI3Kb contribute to tumor proliferation as well. Specifically, it has an important role in tumorigenesis in PTEN-negative cancers.[12] It’s reported that interfering with the gene for PI3Kb might be a therapeutic approach for high-risk bladder cancers with mutant PTEN and E-cadherin loss. Specific isoform inhibitors to PI3Kb is a potential treatment for PTEN-deficient cancers.[13]
Therapies
PI3K inhibitor
PI3K inhibitors may overcome drug resistance and improve advanced breast cancer (ABC) outcomes.[14] Different PI3K inhibitors exhibit different effect against various PI3K types. Class IA pan-PI3K inhibitors have been more extensively studied than isoform specific inhibitors; Pictilisib is another pan-PI3K inhibitor with greater subunitα-inhibitor activity than buparlisib.[15] Idelalisib is the first PI3K inhibitor approved by the US Food and Drug Administration and is utilized in the treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma and follicular lymphoma. Copanlisib is approved for relapsed follicular lymphoma in patients who have received at least two prior systemic therapies.[16]
Akt inhibitor
AKT is downstream to PI3K and is inhibited by Ipatasertib.[17] Akt is an AGC-family kinase and a central, integral signaling node of the PAM pathway. There are three Akt isozymes, Akt1, Akt2 and Akt3. Small-molecule inhibitors of Akt1 could be especially useful to target tumors with a high prevalence of Akt1 E17K activating mutations, which is observed in 4–6% of breast cancers and 1–2% of colorectal cancer.[18] Research towards Akt inhibition has focused on inhibition of two distinct binding sites: (1) the allosteric pocket of the inactive enzyme, and (2) the ATP binding site. Allosteric Akt inhibitors, highlighted by MK-2206, have been extensively evaluated in a clinical setting; Recently, additional allosteric Akt inhibitors have been identified. ARQ-092, is a potent pan-Akt inhibitor which can inhibit tumor growth preclinically and is currently in Phase I clinical studies.[19]
mTOR inhibitor
There is significant correlation of phosphorylated mTOR with the survival rate for patients with stages I and II TNBC. A patient-derived xerograph TNBC model testing the mTOR inhibitor rapamycin showed 77–99% tumor-growth inhibition, which is significantly more than that has been seen with doxorubicin; protein phosphorylation studies indicated that constitutive activation of the mTOR pathway decreased with treatment.[20]
Dual PI3K/AKT/mTOR inhibitors
Here is a hypothesis that blockade of the PI3K/AKT/mTOR pathway can lead to increased antitumor activity in TNBC. Preclinical data have shown that the combination of compounds targeting different cognate molecules in the PI3K/AKT/mTOR pathway leads to synergistic activity. On the basis of these findings, new compounds targeting different components of the PI3K/AKT/mTOR pathway simultaneously continue to be developed. For example, gedatolisib inhibits mutant forms of PI3K-α with elevated kinase activity at concentrations equivalent to the IC50 for wild-type PI3K-α. PI3K-β, -δ and -γ isoforms were inhibited by gedatolisib at concentrations approximately 10-fold higher than those observed for PI3K-α.[21] Another advantage of simultaneously targeting PI3K and mTOR is the ensuing more robust inhibition of receptor tyrosine kinase-positive feedback loops seen with isolated PI3K inhibition.[22] Gedatolisib is currently under development for the treatment of TNBC, in combination with PTK7 antibody–drug conjugate. Apitolisib (GDC-0980) is a PI3K inhibitor (subunits α, δ, and γ) that also targets mTORC [23]
Neural stem cells
The type of growth factor signaling can effect whether or not NSCs differentiate into motor neurons or not. Priming a media with FGF2 lowers the activity of the PI3K/AKT pathway, which activates GSK3β. This increases expression of HB9.[5] Directly inhibiting PI3K in NSCs leads to a population of cells that are purely HB9+ and differentiate at an elevated efficiency into motor neurons. Grafting these cells into different parts of rats generates motor neurons regardless of the transplanted cells' microenvironment.[5] Following injury, neural stem cells enter a repair phase and express high levels of PI3K to enhance proliferation. This is better for survival of the neurons as a whole but is at the expense of generating motor neurons. Therefore, it can be difficult for injured motor neurons to recover their ability.[5] It is the purpose of modern research to generate neural stem cells that can proliferate but still differentiate into motor neurons. Lowering the effect of the PI3K pathway and increasing the effect of GSK3β and HB9 in NSCs is a potential way of generating these cells.[5]
Bisperoxovanadium eg for neuroprotection after CNS injury
PTEN is a natural inhibitor of the PI3K/AKT pathway. Bisperoxovanadium is a specific inhibitor of PTEN's phosphatase activity[24] and has a definite half life. Therefore, by administering this PTEN inhibitor, one can temporarily and safely effect the PI3K/AKT pathway to influence cell migration,[25] survival[26] and proliferation.[6] Too much inhibition leads to unregulated cell cycle progression and tumorigenesis. However, enough PTEN inhibition promotes neuroprotection after CNS injury[27] and leads to an enhanced recovery to the CNS via axonal outgrowth.[6] Axons need to undergo outgrowth so that they can travel and connect to their targets, but lack the intrinsic capability to do so alone. Amplifying the PI3K/AKT pathway increases this neural outgrowth. It is the purpose of modern research to determine appropriate treatment concentrations of bisperoxovanadium to stimulate axonal outgrowth but not cause cancer.[6]
Long-term potentiation
In order for long-term potentiation (LTP) to occur, there must be stimulation of NMDA receptors, which causes AMPA receptors to be inserted postsynaptically. PI3K binds to AMPA receptors in a conserved region to orient the receptors in the membrane, specifically at the GluR subunit.[4] PI3K activity increases in response to calcium ions and CaM. Additionally, AKT localizes PtdIns-3Ps in the post synapse, which recruits docking proteins such as tSNARE and Vam7. This directly leads to the docking of AMPA in the post synapse.[4] mTOR activated p70S6K and inactivated 4EBP1 which changes gene expression to allow LTP to occur.[28] Long-term fear conditioning training was affected in rats but there was no effect in short term conditioning. Specifically, amygdala fear conditioning was lost. This is a type of trace conditioning which is a form of learning that requires association of a conditioned stimulus with an unconditioned stimulus. This effect was lost in PI3K knockdowns and increased in PI3K overexpressions.[29]
Role in brain growth
It is known that PI3K-AKT signaling pathway has an important role in brain growth. In fact, it was discovered that it is the cause of many brain overgrowth disorders. However, this role has not only been described under pathological conditions, but under normal conditions, too. Intracranial volume is also associated with this pathway, in particular with AKT3 intronic variants.[30]
See also
References
- ↑ King, D; Yeomanson, D; Bryant, HE (24 March 2015). "PI3King the Lock: Targeting the PI3K/Akt/mTOR Pathway as a Novel Therapeutic Strategy in Neuroblastoma". Journal of pediatric hematology/oncology. 37 (4): 245–51. doi:10.1097/MPH.0000000000000329. PMID 25811750.
- 1 2 3 4 5 6 7 8 9 Peltier, J; O'Neill, A; Schaffer, D. V. (2007). "PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation". Developmental Neurobiology. 67 (10): 1348–61. doi:10.1002/dneu.20506. PMID 17638387.
- 1 2 3 4 5 6 7 8 9 10 11 Rafalski, V. A.; Brunet, A (2011). "Energy metabolism in adult neural stem cell fate". Progress in Neurobiology. 93 (2): 182–203. doi:10.1016/j.pneurobio.2010.10.007. PMID 21056618.
- 1 2 3 4 5 Man, H. Y.; Wang, Q; Lu, W. Y.; Ju, W; Ahmadian, G; Liu, L; d'Souza, S; Wong, T. P.; Taghibiglou, C; Lu, J; Becker, L. E.; Pei, L; Liu, F; Wymann, M. P.; MacDonald, J. F.; Wang, Y. T. (2003). "Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons". Neuron. 38 (4): 611–24. doi:10.1016/s0896-6273(03)00228-9. PMID 12765612.
- 1 2 3 4 5 6 Ojeda, L; Gao, J; Hooten, K. G.; Wang, E; Thonhoff, J. R.; Dunn, T. J.; Gao, T; Wu, P (2011). "Critical role of PI3K/Akt/GSK3β in motoneuron specification from human neural stem cells in response to FGF2 and EGF". PLoS ONE. 6 (8): e23414. doi:10.1371/journal.pone.0023414. PMC 3160859. PMID 21887250.
- 1 2 3 4 Wyatt, L. A.; Filbin, M. T.; Keirstead, H. S. (2014). "PTEN inhibition enhances neurite outgrowth in human embryonic stem cell-derived neuronal progenitor cells". Journal of Comparative Neurology. 522 (12): 2741–55. doi:10.1002/cne.23580. PMID 24610700.
- ↑ Sui, L; Wang, J; Li, B. M. (2008). "Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex". Learning & Memory. 15 (10): 762–76. doi:10.1101/lm.1067808. PMID 18832563.
- ↑ Aziz, AUR; Farid, S; Qin, K; Wang, H; Liu, B (4 Feb 2018). "PIM Kinases and Their Relevance to the PI3K/AKT/mTOR Pathway in the Regulation of Ovarian Cancer". Biomolecules. doi:10.3390/biom8010007. PMID 29401696.
- ↑ Raphael, Jacques; Desautels, Danielle (March 2018). "Phosphoinositide 3-kinase inhibitors in advanced breast cancer: A systematic review and meta-analysis". European Journal of Cancer. 91: 38–46. doi:10.1016/j.ejca.2017.12.010.
- ↑ Costa, Ricardo L. B.; Han, Hyo Sook; Gradishar, William J (29 January 2018). "Targeting the PI3K/AKT/mTOR pathway in triple‑negative breast cancer: a review". Breast Cancer Research and Treatment. doi:10.1007/s10549-018-4697-y.
- ↑ Serra, V; Markman, B; Scaltriti, M (September 30, 2008). "a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations". Cancer Research. doi:10.1158/0008-5472.CAN-08-1385.
- ↑ Liu, Sandy T.; Hui, Gavin; Mathis, Colleen; Chamie, Karim (April 2018). "The Current Status and Future Role of the Phosphoinositide 3 Kinase/AKT Signaling Pathway in Urothelial Cancer: An Old Pathway in the New Immunotherapy Era". Clin Genitourin Cancer. doi:10.1016/j.clgc.2017.10.011.
- ↑ Winkler, DG; Faia, KL; DiNitto, JP (2013). "PI3K-d and PI3K-g inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models". Chem Biol.
- ↑ Raphael, Jacques; Desautels, Danielle (March 2018). "Phosphoinositide 3-kinase inhibitors in advanced breast cancer: A systematic review and meta-analysis". European Journal of Cancer. 91: 38–46. doi:10.1016/j.ejca.2017.12.010.
- ↑ Costa, Ricardo L. B.; Han, Hyo Sook; Gradishar, William J (29 January 2018). "Targeting the PI3K/AKT/mTOR pathway in triple‑negative breast cancer: a review". Breast Cancer Research and Treatment. doi:10.1007/s10549-018-4697-y.
- ↑ Greenwell, IB; Ip, A; Cohen, JB (15 Nov 017). "PI3K Inhibitors: Understanding Toxicity Mechanisms and Management". Oncology Journal. 31. Check date values in:
|date=(help) - ↑ Costa, Ricardo L. B.; Han, Hyo Sook; Gradishar, William J (29 January 2018). "Targeting the PI3K/AKT/mTOR pathway in triple‑negative breast cancer: a review". Breast Cancer Research and Treatment. doi:10.1007/s10549-018-4697-y.
- ↑ Huck, Bayard R; Mochalkin, Igor (1 July 2017). "Recent progress towards clinically relevant ATP-competitive Akt inhibitors". Bioorganic & Medicinal Chemistry Letters. 27: 2838–2848. doi:10.1016/j.bmcl.2017.04.090.
- ↑ Huck, Bayard R; Mochalkin, Igor (1 July 2017). "Recent progress towards clinically relevant ATP-competitive Akt inhibitors". Bioorganic & Medicinal Chemistry Letters. 27: 2838–2848. doi:10.1016/j.bmcl.2017.04.090.
- ↑ Costa, Ricardo L. B.; Han, Hyo Sook; Gradishar, William J (29 January 2018). "Targeting the PI3K/AKT/mTOR pathway in triple‑negative breast cancer: a review". Breast Cancer Research and Treatment. doi:10.1007/s10549-018-4697-y.
- ↑ Costa, Ricardo L. B.; Han, Hyo Sook; Gradishar, William J (29 January 2018). "Targeting the PI3K/AKT/mTOR pathway in triple‑negative breast cancer: a review". Breast Cancer Research and Treatment. doi:10.1007/s10549-018-4697-y.
- ↑ Chakrabarty, A; Sanchez, V; Kuba, MG; Rinehart, C; Arteaga, CL (2012). "Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors". Proc Natl Acad Sci USA. doi:10.1073/pnas.1018001108. PMC 3286932.
- ↑ Cappellen, D; Gil Diez de Medina, S; Chopin, D (1997). "Frequent loss of heterozygosity on chromosome 10q in muscle-invasive transitional cell carcinomas of the bladder". Oncogene. doi:10.1038/sj.onc.1201154.
- ↑ Schmid, A. C.; Byrne, R. D.; Vilar, R; Woscholski, R (2004). "Bisperoxovanadium compounds are potent PTEN inhibitors". FEBS Letters. 566 (1–3): 35–8. doi:10.1016/j.febslet.2004.03.102. PMID 15147864.
- ↑ Mihai, C; Bao, S; Lai, J. P.; Ghadiali, S. N.; Knoell, D. L. (2012). "PTEN inhibition improves wound healing in lung epithelia through changes in cellular mechanics that enhance migration". AJP: Lung Cellular and Molecular Physiology. 302 (3): L287–99. doi:10.1152/ajplung.00037.2011. PMC 3289272. PMID 22037358.
- ↑ Lai, J. P.; Dalton, J. T.; Knoell, D. L. (2007). "Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair". British Journal of Pharmacology. 152 (8): 1172–84. doi:10.1038/sj.bjp.0707501. PMC 2189995. PMID 17922022.
- ↑ Walker, C. L.; Walker, M. J.; Liu, N. K.; Risberg, E. C.; Gao, X; Chen, J; Xu, X. M. (2012). "Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury". PLoS ONE. 7 (1): e30012. doi:10.1371/journal.pone.0030012. PMC 3254642. PMID 22253859.
- ↑ Sui, L; Wang, J; Li, B. M. (2008). "Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex". Learning & Memory. 15 (10): 762–76. doi:10.1101/lm.1067808. PMID 18832563.
- ↑ Sui, L; Wang, J; Li, B. M. (2008). "Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex". Learning & Memory. 15 (10): 762–76. doi:10.1101/lm.1067808. PMID 18832563.
- ↑ Adams, Hieab H H; Hibar, Derrek P; Chouraki, Vincent; Stein, Jason L; Nyquist, Paul A; Rentería, Miguel E; Trompet, Stella; Arias-Vasquez, Alejandro; Seshadri, Sudha (2016). "Novel genetic loci underlying human intracranial volume identified through genome-wide association". Nature Neuroscience. 19 (12): 1569–1582. doi:10.1038/nn.4398. PMC 5227112. PMID 27694991.