Orotate phosphoribosyltransferase
| Orotate phosphoribosyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
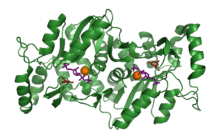 S. cerevisiae orotate phosphoribosyl transferase bound to its substrates. From PDB file 2PS1.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 2.4.2.10 | ||||||||
| CAS number | 9030-25-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Orotate phosphoribosyltransferase (OPRTase) or orotic acid phosphoribosyltransferase is an enzyme involved in pyrimidine biosynthesis. It catalyzes the formation of orotidine 5'-monophosphate (OMP) from orotate and phosphoribosyl pyrophosphate.[1] In yeast and bacteria, orotate phosphoribosyltransferase is an independent enzyme with a unique gene coding for the protein, whereas in mammals and other multicellular organisms, the catalytic function is carried out by a domain of the bifunctional enzyme UMP synthase.[2]
Biological background
As OPRTase is part of a bifunctional complex UMP synthase in humans, the function and stability of this enzyme is not necessarily directly associated with disorders in the human body. It is however reasonable to believe that a dysfunction in one of the enzymes will cause a dysfunction of the whole enzyme.[3] Defects in UMP synthase is associated with hypochromic anemia.[3]
Protein structure and function
The crystal structure of OPRTase has been solved several times by various scientific groups.[1][4][5][6] The overall structure is a dimer of two subunits, each consisting of seven α-helices and ten β-strands. The active site can be closed by a flexible loop, that prevents solvent from entering during reaction.[1]
References
- 1 2 3 4 González-Segura L, Witte JF, McClard RW, Hurley TD (December 2007). "Ternary complex formation and induced asymmetry in orotate phosphoribosyltransferase". Biochemistry. 46 (49): 14075–86. doi:10.1021/bi701023z. PMID 18020427.
- ↑ Yablonski MJ, Pasek DA, Han BD, Jones ME, Traut TW (1996). "Intrinsic activity and stability of bifunctional human UMP synthase and its two separate catalytic domains, orotate phosphoribosyltransferase and orotidine-5'-phosphate decarboxylase". J Biol Chem. 271 (18): 10704–10708. doi:10.1074/jbc.271.18.10704. PMID 8631878.
- 1 2 Musick DL. (1981). "Structural Features of the Phosphoribosyltransferases and Their Relationship to the Human Deficiency Disorders of Purine and Pyrimidine Metabolism". Critical Reviews in Biochemistry and Molecular Biology. 11 (June): 1–34. doi:10.3109/10409238109108698. PMID 7030616.
- ↑ Grubmeyer C, Hansen MR, Fedorov AA, Almo SC (2012). "Structure of Salmonella typhimurium OMP Synthase in a Complete Substrate Complex". Biochemistry. 51: 4397–4405. doi:10.1021/bi300083p. PMC 3442144. PMID 22531064.
- ↑ Henriksen A, Aghajari N, Jensen KF, Gajhede M (1996). "A Flexible Loop at the Dimer Interface Is a Part of the Active Site of the Adjacent Monomer of Escherichia Coli Orotate Phosphoribosyltransferase". Biochemistry. 35 (12): 3803–3809. doi:10.1021/bi952226y. PMID 8620002.
- ↑ Scapin G, Grubmeyer C, Sacchettini JC (1994). "Crystal Structure of Orotate Phosphoribosyltransferase". Biochemistry. 33 (6): 1287–1294. doi:10.1021/bi00172a001.