Activation energy

In chemistry and physics, activation energy is the energy which must be available to a chemical or nuclear[1] system with potential reactants to result in: a chemical reaction[2], nuclear reaction[3], or various other physical phenomena.[4][5]
The activation energy (Ea) of a reaction is measured in joules (J) and or kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol).[6]
Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction, or division [7] to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy.
The term Activation Energy was introduced in 1889 by the Swedish scientist Svante Arrhenius.[8]
Temperature dependence and the relation to the Arrhenius equation
The Arrhenius equation gives the quantitative basis of the relationship between the activation energy and the rate at which a reaction proceeds. From the equation, the activation energy can be found through the relation
where A is the pre-exponential factor for the reaction, R is the universal gas constant, T is the absolute temperature (usually in kelvins), and k is the reaction rate coefficient. Even without knowing A, Ea can be evaluated from the variation in reaction rate coefficients as a function of temperature (within the validity of the Arrhenius equation).
At a more advanced level, the net Arrhenius activation energy term from the Arrhenius equation is best regarded as an experimentally determined parameter that indicates the sensitivity of the reaction rate to temperature. There are two objections to associating this activation energy with the threshold barrier for an elementary reaction. First, it is often unclear as to whether or not reaction does proceed in one step; threshold barriers that are averaged out over all elementary steps have little theoretical value. Second, even if the reaction being studied is elementary, a spectrum of individual collisions contributes to rate constants obtained from bulk ('bulb') experiments involving billions of molecules, with many different reactant collision geometries and angles, different translational and (possibly) vibrational energies—all of which may lead to different microscopic reaction rates.
Negative activation energy
In some cases, rates of reaction decrease with increasing temperature. When following an approximately exponential relationship so the rate constant can still be fit to an Arrhenius expression, this results in a negative value of Ea. Elementary reactions exhibiting these negative activation energies are typically barrierless reactions, in which the reaction proceeding relies on the capture of the molecules in a potential well. Increasing the temperature leads to a reduced probability of the colliding molecules capturing one another (with more glancing collisions not leading to reaction as the higher momentum carries the colliding particles out of the potential well), expressed as a reaction cross section that decreases with increasing temperature. Such a situation no longer leads itself to direct interpretations as the height of a potential spot.
Catalysts
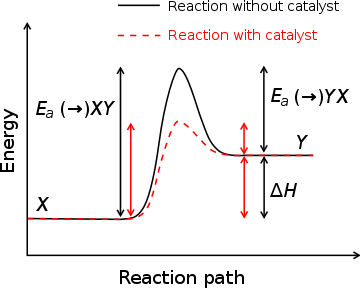
A substance that modifies the transition state to lower the activation energy is termed a catalyst; a catalyst composed only of protein and (if applicable) small molecule cofactors is termed an enzyme. It is important to note that a catalyst increases the rate of reaction without being consumed by it.[9] In addition, while the catalyst lowers the activation energy, it does not change the energies of the original reactants or products.[10] Rather, the reactant energy and the product energy remain the same and only the activation energy is altered (lowered).
Relationship with Gibbs energy
In the Arrhenius equation, the term activation energy (Ea) is used to describe the energy required to reach the transition state. Likewise, the Eyring equation is a similar equation that also describes the rate of a reaction. Instead of also using Ea, however, the Eyring equation uses the concept of Gibbs energy and the symbol * to denote the energy of the transition state. This implies that the equation is similar but not identical to the Arrhenius one, because the Gibbs energy contains an entropic term in addition to the enthalpic one.
See also
References
- ↑ http://www.physics.ohio-state.edu/~kagan/phy367/Lectures/P367_lec_14.html%5Bfull+citation+needed%5D
- ↑ "Activation Energy". www.chem.fsu.edu. Retrieved 2017-01-13.
- ↑ http://www.physics.ohio-state.edu/~kagan/phy367/Lectures/P367_lec_14.html
- ↑ Wang, Jenqdaw; Raj, Rishi (1990). "Estimate of the Activation Energies for Boundary Diffusion from Rate-Controlled Sintering of Pure Alumina, and Alumina Doped with Zirconia or Titania". Journal of the American Ceramic Society. 73 (5): 1172. doi:10.1111/j.1151-2916.1990.tb05175.x.
- ↑ Kiraci, A; Yurtseven, H (2012). "Temperature Dependence of the Raman Frequency, Damping Constant and the Activation Energy of a Soft-Optic Mode in Ferroelectric Barium Titanate". Ferroelectrics. 432: 14. doi:10.1080/00150193.2012.707592.
- ↑ Pratt, Thomas H. "Electrostatic Ignitions of Fires and Explosions" Wiley-AIChE (July 15, 1997) Center for Chemical Process Safety
- ↑ Terracciano, Anthony C; De Oliveira, Samuel; Vazquez-Molina, Demetrius; Uribe-Romo, Fernando J; Vasu, Subith S; Orlovskaya, Nina (2017). "Effect of catalytically active Ce 0.8 Gd 0.2 O 1.9 coating on the heterogeneous combustion of methane within MgO stabilized ZrO 2 porous ceramics". Combustion and Flame. 180: 32. doi:10.1016/j.combustflame.2017.02.019.
- ↑ "Activation Energy and the Arrhenius Equation – Introductory Chemistry- 1st Canadian Edition". opentextbc.ca. Retrieved 2018-04-05.
- ↑ "General Chemistry Online: FAQ: Chemical change: What are some examples of reactions that involve catalysts?". antoine.frostburg.edu. Retrieved 2017-01-13.
- ↑ Bui, Matthew. "The Arrhenius Law: Activation Energies". Chemistry LibreTexts. UC Davis. Retrieved February 17, 2017.