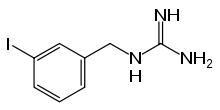
Iobenguane
 | |
| Clinical data | |
|---|---|
| Synonyms |
meta-iodobenzylguanidine mIBG, MIBG |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C8H10IN3 |
| Molar mass | 275.09 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Iobenguane, also known as metaiodobenzylguanidine or mIBG, or MIBG (tradename Adreview) is a radiopharmaceutical,[1] used in a scintigraphy method called MIBG scan. Iobenguane is a radiolabeled molecule similar to noradrenaline.
The radioisotope of iodine used for the label can be iodine-123 (for imaging purposes only) or iodine-131 (which must be used when tissue destruction is desired, but is sometimes used for imaging also).
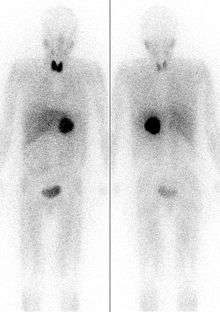
It localizes to adrenergic tissue and thus can be used to identify the location of tumors[2] such as pheochromocytomas and neuroblastomas. With I-131 it can also be used to eradicate tumor cells that take up and metabolize norepinephrine.
Thyroid precautions
Thyroid blockade with (nonradioactive) potassium iodide is indicated for nuclear medicine scintigraphy with iobenguane/mIBG. This competitively inhibits radioiodine uptake, preventing excessive radioiodine levels in the thyroid and minimizing the risk of thyroid ablation ( in the case of I-131). The minimal risk of thyroid carcinogenesis is also reduced as a result.
The FDA-approved dosing of potassium iodide for this purpose are as follows: infants less than 1 month old, 16 mg; children 1 month to 3 years, 32 mg; children 3 years to 18 years, 65 mg; adults 130 mg.[3] However, some sources recommend alternative dosing regimens.[4]
Not all sources are in agreement on the necessary duration of thyroid blockade, although agreement appears to have been reached about the necessity of blockade for both scintigraphic and therapeutic applications of iobenguane. Commercially available iobenguane is labeled with iodine-123, and product labeling recommends administration of potassium iodide 1 hour prior to administration of the radiopharmaceutical for all age groups,[5] while the European Associated of Nuclear Medicine recommends (for iobenguane labeled with either I-131 or I-123,) that potassium iodide administration begin one day prior to radiopharmaceutical administration, and continue until the day following the injection, with the exception of newborns, who do not require potassium iodide doses following radiopharmaceutical injection.[4]
Product labeling for diagnostic iodine-131 iobenguane recommends potassium iodide administration one day before injection and continuing 5 to 7 days following.[6] Iodine-131 iobenguane used for therapeutic purposes requires a different pre-medication duration, beginning 24–48 hours prior to iobenguane injection and continuing 10–15 days following injection.[7]
Alternative imaging modality for pheochromocytoma
The FDOPA PET/CT scan has proven to be nearly 100% sensitive for detection of pheochromocytomas, vs. 90% for MIBG scans.[8][9][10] Centers which offer FDOPA PET/CT, however, are rare.
Clinical trials
Iobenguane I 131 for cancers
Iobenguane I 131, marketed under the trade name Azedra, has had a clinical trial as a treatment for malignant, recurrent or unresectable pheochromocytoma and paraganglioma, and the FDA approved it on July 30th, 2018. The drug is developed by Progenics Pharmaceuticals.[11][12]
References
- ↑ "Iobenguane I 131 Sulfate (Intravenous Route) - MayoClinic.com". Retrieved 2007-12-15.
- ↑ Scarsbrook AF, Ganeshan A, Statham J, et al. (2007). "Anatomic and functional imaging of metastatic carcinoid tumors". Radiographics. 27 (2): 455–77. doi:10.1148/rg.272065058. PMID 17374863.
- ↑ Kowalsky RJ, Falen, SW. Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine. 2nd ed. Washington DC: American Pharmacists Association; 2004.
- 1 2 "Archived copy" (PDF). Archived from the original (PDF) on 2011-10-07. Retrieved 2011-10-07.
- ↑ http://nuclearpharmacy.uams.edu/resources/adreview.pdf
- ↑ Iobenguane Sulfate I 131 Injection Diagnostic package insert. Bedford, MA: CIS-US, Inc. July 1999.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2011-10-07. Retrieved 2011-10-07.
- ↑ 6-[18FFluorodopamine Positron Emission Tomographic (PET) Scanning for Diagnostic Localization of Pheochromocytoma. Pacek et al. 2001] full text
- ↑ Pheochromocytoma Imaging Updated May 2017
- ↑ Luster M, Karges W, Zeich K, Pauls S, Verburg FA, Dralle H; et al. (2010). "Clinical value of (18)F-fluorodihydroxyphenylalanine positron emission tomography/computed tomography ((18)F-DOPA PET/CT) for detecting pheochromocytoma". European journal of nuclear medicine and molecular imaging. 37 (3): 484–93. doi:10.1007/s00259-009-1294-7. PMID 19862519.
- ↑ AZEDRA® (iobenguane I 131) Jul 2018
- ↑ FDA approves first treatment for rare adrenal tumors Jul 2018
External links
- Iodine 131-metaiodobenzylguanidine at the NCI Drug Dictionary