Glutaraldehyde
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pentanedial[1] | |
| Other names
Glutaraldehyde Glutardialdehyde Glutaric acid dialdehyde Glutaric aldehyde Glutaric dialdehyde 1,5-Pentanedial | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.506 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C5H8O2 | |
| Molar mass | 100.117 |
| Appearance | Clear liquid |
| Odor | pungent[2] |
| Density | 1.06 g/mL |
| Melting point | −14 °C (7 °F; 259 K) |
| Boiling point | 187 °C (369 °F; 460 K) |
| Miscible, reacts | |
| Vapor pressure | 17 mmHg (20°C)[2] |
| Hazards | |
| Flash point | noncombustible[2] |
| US health exposure limits (NIOSH): | |
PEL (Permissible) |
none[2] |
REL (Recommended) |
C 0.2 ppm (0.8 mg/m3)[2] |
IDLH (Immediate danger) |
N.D.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glutaraldehyde, sold under the brandname Cidex and Glutaral among others, is a disinfectant and medication.[3][4][5] As a disinfectant it is used to sterilize surgical instruments and other areas.[3] As a medication it is used to treat warts on the bottom of the feet.[4] It is applied as a liquid.[3]
Side effects include skin irritation.[4] If exposed to large amounts, nausea, headache, and shortness of breath may occur.[3] Protective equipment is recommended when used.[3] Glutaraldehyde is effective against a range of microorganisms including spores.[3][6] It works by a number of mechanisms.[6]
Glutaraldehyde came into medical use in the 1960s.[7] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[8] The wholesale cost in the developing world is about 1.50 to 7.40 USD per liter of 2% solution.[9] There are a number of other commercial uses such as leather tanning.[10]
Uses
Disinfectant
Virtually all applications of glutaraldehyde exploit its high reactivity toward proteins. Being nonvolatile and bifunctional, glutaraldehyde is often preferred to the less expensive formaldehyde. It reacts with amines, amides, and thiol groups in proteins.[11]
A glutaraldehyde solution of 0.1% to 1.0% concentration may be used as a biocide for system disinfection and as a preservative for long term storage. It is a sterilant, killing endospores in addition to many microorganisms and viruses.[12]
Glutaraldehyde is a component of hydraulic fracturing ("fracking") fluid. It is included in the additive called Alpha 1427, as a biocide.[13] Bacterial growth can impair the production of oil and gas wells, and can be introduced into the formation from various sources including the source water, proppant, and polymers used in the hydraulic fracturing process. Glutaraldehyde is pumped as a liquid additive with the fracturing fluid to reduce or eliminate this source of formation and fracture conductivity damage.
Fixative
Glutaraldehyde is used in biochemistry applications as an amine-reactive homobifunctional crosslinker and fixative prior to SDS-PAGE, staining, or electron microscopy. It kills cells quickly by crosslinking their proteins and is usually employed alone or mixed with formaldehyde[14] as the first of two fixative processes to vistabilize specimens such as bacteria, plant material, and human cells. A second fixative procedure uses osmium tetroxide to crosslink and stabilize cell and organelle membrane lipids. Fixation is usually followed by dehydration of the tissue in ethanol or acetone, followed by embedding in an epoxy resin or acrylic resin.
Another application for treatment of proteins with glutaraldehyde is the inactivation of bacterial toxins to create toxoid vaccines, e.g., the pertussis (whooping cough) toxoid component in the Boostrix Tdap vaccine produced by GlaxoSmithKline.[15]
In a related application, glutaraldehyde is sometimes employed in the tanning of leather and in embalming.
Wart treatment
A solution of glutaraldehyde, typically of 10% w/w, is sold under various trade names to remove common and plantar warts. It is said to inactivate viruses and bacteria, and to dry the skin, facilitating physical removal of the wart.[16] Trade names include Diswart Solution and Glutarol.
Safety
As a strong sterilant, glutaraldehyde is toxic and a strong irritant.[17] There is no strong evidence of carcinogenic activity.[18] Some occupations that work with this chemical have an increased risk of some cancers.[18]
Chemistry
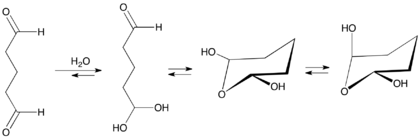
It is an organic compound with the formula OCH(CH2)3CHO. At room temperature it is a pungent colorless oily liquid. It is mainly available as an aqueous solution, and in these solutions the aldehyde groups are hydrated.[19]
Production and structure
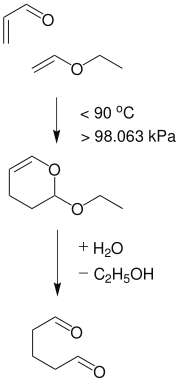
Glutaraldehyde is produced industrially by the oxidation of cyclopentene and by the Diels-Alder reaction of acrolein and methyl vinyl ether followed by hydrolysis.[19]
Like other dialdehydes (e.g., glyoxal), it does not exist as the dialdehyde in water, but as the hydrate. These hydrates adopt several equilibrating species.[20]
Monomeric glutaraldehyde can polymerize by aldol condensation reaction yielding alpha, beta-unsaturated poly-glutaraldehyde. This reaction usually occurs at alkaline pH values.
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 907. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 5 6 "CDC - NIOSH Pocket Guide to Chemical Hazards -Glutaraldehyde". www.cdc.gov. Archived from the original on 13 January 2017. Retrieved 11 January 2017.
- 1 2 3 4 5 6 WHO Model Formulary 2008 (PDF). World Health Organization. 2009. pp. 323, 325. ISBN 9789241547659. Archived (PDF) from the original on 13 December 2016. Retrieved 8 January 2017.
- 1 2 3 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 825. ISBN 9780857111562.
- ↑ Bonewit-West, Kathy (2015). Clinical Procedures for Medical Assistants. Elsevier Health Sciences. p. 96. ISBN 9781455776610. Archived from the original on 2017-09-23.
- 1 2 Fraise, Adam P.; Maillard, Jean-Yves; Sattar, Syed (2012). Russell, Hugo and Ayliffe's Principles and Practice of Disinfection, Preservation and Sterilization. John Wiley & Sons. p. Chapter 2. ISBN 9781118425862. Archived from the original on 2017-09-23.
- ↑ Booth, Anne (1998). Sterilization of Medical Devices. CRC Press. p. 8. ISBN 9781574910872. Archived from the original on 2017-09-23.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ↑ "Glutaraldehyde". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ↑ Rietschel, Robert L.; Fowler, Joseph F.; Fisher, Alexander A. (2008). Fisher's Contact Dermatitis. PMPH-USA. p. 359. ISBN 9781550093780. Archived from the original on 2017-09-23.
- ↑ H. Uhr, B. Mielke, O. Exner, K. R. Payne, E. Hill (2013), "Biocides", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a16_563.pub2
- ↑ HCC lecture notes, 15 Archived 2015-05-02 at the Wayback Machine.: Control of microorganisms Archived 2015-09-24 at the Wayback Machine.
- ↑ Morgantown Utility Board. "Fracking Fluid Additives - Fracking Fluid MSDS's". Archived from the original on 2015-02-05. . Links to documents, including Alpha 1427 Material Safety Data Sheet
- ↑ Karnovsky, M.J. (1965). A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology 27: 137A–138A
- ↑ Boostrix prescribing information Archived 2011-02-01 at the Wayback Machine., ©2009, GlaxoSmithKline
- ↑ NHS Choices: Glutarol Archived 2015-02-05 at the Wayback Machine.
- ↑ Canadian Centre for Occupational Health and Safety (CCOHS) (a federal government site) > OSH Answers > Diseases, Disorders & Injuries > Asthma Archived 2009-04-27 at the Wayback Machine. Document last updated on February 8, 2005
- 1 2 Toxicology and Carcinogenesis Studies of Glutaraldehyde Archived 2012-10-10 at the Wayback Machine.
- 1 2 Christian Kohlpaintner, Markus Schulte, Jürgen Falbe, Peter Lappe, Jürgen Weber, "Aldehydes, Aliphatic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a01_321.pub2
- ↑ Whipple Earl B.; Ruta Michael (1974). "Structure of Aqueous Glutaraldehyde". J. Org. Chem. 39: 1666–1668. doi:10.1021/jo00925a015.