Extremophile
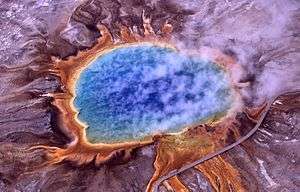
An extremophile (from Latin extremus meaning "extreme" and Greek philiā (φιλία) meaning "love") is an organism that thrives in physically or geochemically extreme conditions that are detrimental to most life on Earth.[1][2] In contrast, organisms that live in more moderate environments may be termed mesophiles or neutrophiles.
Characteristics
In the 1980s and 1990s, biologists found that microbial life has great flexibility for surviving in extreme environments—niches that are acidic or extraordinarily hot, for example—that would be completely inhospitable to complex organisms. Some scientists even concluded that life may have begun on Earth in hydrothermal vents far under the ocean's surface.[3]
According to astrophysicist Steinn Sigurdsson, "There are viable bacterial spores that have been found that are 40 million years old on Earth—and we know they're very hardened to radiation."[4] Some bacteria were found living in the cold and dark in a lake buried a half-mile deep under the ice in Antarctica,[5] and in the Marianas Trench, the deepest place in Earth's oceans.[6][7] Some microorganisms have been found thriving inside rocks up to 1,900 feet (580 m) below the sea floor under 8,500 feet (2,600 m) of ocean off the coast of the northwestern United States.[6][8] According to one of the researchers, "You can find microbes everywhere—they're extremely adaptable to conditions, and survive wherever they are."[6] A key to extremophile adaptation is their amino acid composition, affecting their protein folding ability under particular conditions.[9]
| The limits of known life on Earth.[10] | |||
|---|---|---|---|
| Factor | Environment / source | Limits | Examples |
| High temperature | Submarine hydrothermal vents | 110 °C to 121 °C | Pyrolobus fumarii, Pyrococcus furiosus |
| Low temperature | Ice | -17 °C to -20 °C | Synechococcus lividus |
| Alkaline systems | Soda lakes | pH > 11 | Psychrobacter, Vibrio, Arthrobacter, Natronobacterium |
| Acidic systems | Volcanic springs, acid mine drainage | pH -0.06 to 1.0 | Bacillus, Clostridium paradoxum |
| Ionizing radiation | Cosmic rays, X-rays, radioactive decay | 1,500 to 6,000 Gy | Deinococcus radiodurans, Rubrobacter, Thermococcus gammatolerans |
| UV radiation | Sunlight | 5,000 J/m2 | Deinococcus radiodurans, Rubrobacter, Thermococcus gammatolerans |
| High pressure | Mariana Trench | 1,100 bar | Pyrococcus sp. |
| Salinity | High salt concentration | aw ~ 0.6 | Halobacteriaceae, Dunaliella salina |
| Desiccation | Atacama Desert (Chile), McMurdo Dry Valleys (Antarctica) | ~60% relative humidity | Chroococcidiopsis |
Classifications
There are many classes of extremophiles that range all around the globe, each corresponding to the way its environmental niche differs from mesophilic conditions. These classifications are not exclusive. Many extremophiles fall under multiple categories and are classified as polyextremophiles. For example, organisms living inside hot rocks deep under Earth's surface are thermophilic and barophilic such as Thermococcus barophilus.[11] A polyextremophile living at the summit of a mountain in the Atacama Desert might be a radioresistant xerophile, a psychrophile, and an oligotroph. Polyextremophiles are well known for their ability to tolerate both high and low pH levels.[12]
Terms
- Acidophile
- An organism with optimal growth at pH levels of 3 or below
- Alkaliphile
- An organism with optimal growth at pH levels of 9 or above
- Anaerobe
- An organism that does not require oxygen for growth such as Spinoloricus cinzia. Two sub-types exist: facultative anaerobe and obligate anaerobe. A facultative anaerobe can tolerate anaerobic and aerobic conditions; however, an obligate anaerobe would die in the presence of even trace levels of oxygen
- Cryptoendolith
- An organism that lives in microscopic spaces within rocks, such as pores between aggregate grains; these may also be called endolith, a term that also includes organisms populating fissures, aquifers, and faults filled with groundwater in the deep subsurface
- Hyperthermophile
- An organism that can thrive at temperatures above 80 °C, such as those found in hydrothermal systems
- Hypolith
- An organism that lives underneath rocks in cold deserts
- Lithoautotroph
- An organism (usually bacteria) whose sole source of carbon is carbon dioxide and exergonic inorganic oxidation (chemolithotrophs) such as Nitrosomonas europaea; these organisms are capable of deriving energy from reduced mineral compounds like pyrites, and are active in geochemical cycling and the weathering of parent bedrock to form soil
- Metallotolerant
- Capable of tolerating high levels of dissolved heavy metals in solution, such as copper, cadmium, arsenic, and zinc; examples include Ferroplasma sp., Cupriavidus metallidurans and GFAJ-1[14][15][16]
- Oligotroph
- An organism capable of growth in nutritionally limited environments
- Osmophile
- An organism capable of growth in environments with a high sugar concentration
- Piezophile
- Also referred to as barophile, is an organism that lives optimally at high pressures such as those deep in the ocean or underground;[17] common in the deep terrestrial subsurface, as well as in oceanic trenches. Piezophilic organisms live under conditions of extreme pressure. High pressures can cause proteins to fold into themselves. Piezophiles have less large bulky amino acids that would take up space and prevent the other amino acids from coming close enough to create the reinforced area around the core.[9]
- Polyextremophile
- A polyextremophile (faux Ancient Latin/Greek for 'affection for many extremes') is an organism that qualifies as an extremophile under more than one category
- Psychrophile/Cryophile
- An organism capable of survival, growth or reproduction at temperatures of -15 °C or lower for extended periods; common in cold soils, permafrost, polar ice, cold ocean water, and in or under alpine snowpack. Psychrophilic organisms live in environments at temperature below -15 °C. Low temperatures cause the energy within proteins to slow, which prevents them from functioning. Their proteins have adapted their amino acid composition to live in cold conditions. They have a high amount of glycine amino acids, which small size allows for more flexibility within the protein once it is folded. Psychrophiles also have a low concentration of charged amino acids, hydrophobic (non-polar) amino acids, and proline residues. The low amount of charged amino acids reduces the amount of interactions between them, while the reduced amount of hydrophobic (non-polar) amino acids allows the non-polar core of the protein to be smaller. Psychrophilic proteins have a small amount of proline amino acids because they cause a rigid structure. These adaptations allow psychrophilic proteins to be more flexible so they do not freeze under cold conditions.[9]
- Radioresistant
- Organisms resistant to high levels of ionizing radiation, most commonly ultraviolet radiation, but also including organisms capable of resisting nuclear radiation
- Thermophile
- An organism that can thrive at temperatures between 45–122 °C. Normally high temperatures cause proteins to unfold and prevent them from functioning; thermophilic proteins have adapted their proteins to cope with these conditions. They have a higher amount of cysteine amino acids, which form disulfide bonds once the protein has folded. These disulfide bonds cause the protein to fold up tighter and become more rigid. They also have more charged amino acids, both positive and negative, that interact with each other to increase how rigid the protein is. When proteins become more rigid they are less likely to unfold due to high temperature. Even one extra disulfide bond can raise the temperature at which the protein folds by 6 °C. These adapted proteins allow thermophilic extremophiles to survive at high temperatures.[9]
- Thermoacidophile
- Combination of thermophile and acidophile that prefer temperatures of 70–80 °C and pH between 2 and 3
- Xerophile
- An organism that can grow in extremely dry, desiccating conditions; this type is exemplified by the soil microbes of the Atacama Desert
In astrobiology
Astrobiology is the study of the origin, evolution, distribution, and future of life in the universe: extraterrestrial life and life on Earth. Astrobiology makes use of physics, chemistry, astronomy, solar physics, biology, molecular biology, ecology, planetary science, geography, and geology to investigate the possibility of life on other worlds and help recognize biospheres that might be different from that on Earth.[18] Astrobiologists are particularly interested in studying extremophiles,[19] as their habitats may be analogous to conditions on other planets. For example, analogous deserts of Antarctica are exposed to harmful UV radiation, low temperature, high salt concentration and low mineral concentration. These conditions are similar to those on Mars. Therefore, finding viable microbes in the subsurface of Antarctica suggests that there may be microbes surviving in endolithic communities and living under the Martian surface. Research indicates it is unlikely that Martian microbes exist on the surface or at shallow depths, but may be found at subsurface depths of around 100 meters.[20]
Recent research carried out on extremophiles in Japan involved a variety of bacteria including Escherichia coli and Paracoccus denitrificans being subject to conditions of extreme gravity. The bacteria were cultivated while being rotated in an ultracentrifuge at high speeds corresponding to 403,627 g (i.e. 403,627 times the gravity experienced on Earth). Paracoccus denitrificans was one of the bacteria which displayed not only survival but also robust cellular growth under these conditions of hyperacceleration which are usually found only in cosmic environments, such as on very massive stars or in the shock waves of supernovas. Analysis showed that the small size of prokaryotic cells is essential for successful growth under hypergravity. The research has implications on the feasibility of panspermia.[21][22]
On 26 April 2012, scientists reported that lichen survived and showed remarkable results on the adaptation capacity of photosynthetic activity within the simulation time of 34 days under Martian conditions in the Mars Simulation Laboratory (MSL) maintained by the German Aerospace Center (DLR).[23][24]
On 29 April 2013, scientists at Rensselaer Polytechnic Institute, funded by NASA, reported that, during spaceflight on the International Space Station, microbes seem to adapt to the space environment in ways "not observed on Earth" and in ways that "can lead to increases in growth and virulence".[25]
On 19 May 2014, scientists announced that numerous microbes, like Tersicoccus phoenicis, may be resistant to methods usually used in spacecraft assembly clean rooms. It's not currently known if such resistant microbes could have withstood space travel and are present on the Curiosity rover now on the planet Mars.[26]
On 20 August 2014, scientists confirmed the existence of microorganisms living half a mile below the ice of Antarctica.[27][28]
On September 2015, scientists from CNR-National Research Council of Italy reported that S.soflataricus was able to survive under Martian radiation at a wavelength that was considered extremely lethal to most bacteria. This discovery is significant because it indicates that not only bacterial spores, but also growing cells can be remarkably resistant to strong UV radiation.[29]
On June 2016, scientists from Brigham Young University conclusively reported that endospores of Bacillus subtilis were able to survive high speed impacts up to 299±28 m/s, extreme shock, and extreme deceleration. They pointed out that this feature might allow endospores to survive and to be transferred between planets by traveling within meteorites or by experiencing atmosphere disruption. Moreover, they suggested that the landing of spacecraft may also result in interplanetary spore transfer, given that spores can survive high-velocity impact while ejected from the spacecraft onto the planet surface. This is the first study which reported that bacteria can survive in such high-velocity impact. However, the lethal impact speed is unknown, and further experiments should be done by introducing higher-velocity impact to bacterial endospores.[30]
Examples
New sub-types of -philes are identified frequently and the sub-category list for extremophiles is always growing. For example, microbial life lives in the liquid asphalt lake, Pitch Lake. Research indicates that extremophiles inhabit the asphalt lake in populations ranging between 106 to 107 cells/gram.[31][32] Likewise, until recently boron tolerance was unknown but a strong borophile was discovered in bacteria. With the recent isolation of Bacillus boroniphilus, borophiles came into discussion.[33] Studying these borophiles may help illuminate the mechanisms of both boron toxicity and boron deficiency.
Industrial uses
The thermoalkaliphilic catalase, which initiates the breakdown of hydrogen peroxide into oxygen and water, was isolated from an organism, Thermus brockianus, found in Yellowstone National Park by Idaho National Laboratory researchers. The catalase operates over a temperature range from 30 °C to over 94 °C and a pH range from 6–10. This catalase is extremely stable compared to other catalases at high temperatures and pH. In a comparative study, the T. brockianus catalase exhibited a half life of 15 days at 80 °C and pH 10 while a catalase derived from Aspergillus niger had a half life of 15 seconds under the same conditions. The catalase will have applications for removal of hydrogen peroxide in industrial processes such as pulp and paper bleaching, textile bleaching, food pasteurization, and surface decontamination of food packaging.[34]
DNA modifying enzymes such as Taq DNA polymerase and some Bacillus enzymes used in clinical diagnostics and starch liquefaction are produced commercially by several biotechnology companies.[35]
DNA transfer
Over 65 prokaryotic species are known to be naturally competent for genetic transformation, the ability to transfer DNA from one cell to another cell followed by integration of the donor DNA into the recipient cell’s chromosome.[36] Several extremophiles are able to carry out species-specific DNA transfer, as described below. However, it is not yet clear how common such a capability is among extremophiles.
The bacterium Deinococcus radiodurans is one of the most radioresistant organisms known. This bacterium can also survive cold, dehydration, vacuum and acid and is thus known as a polyextremophile. D. radiodurans is competent to perform genetic transformation.[37] Recipient cells are able to repair DNA damage in donor transforming DNA that had been UV irradiated as efficiently as they repair cellular DNA when the cells themselves are irradiated. The extreme thermophilic bacterium Thermus thermophilus and other related Thermus species are also capable of genetic transformation.[38]
Halobacterium volcanii, an extreme halophilic (saline tolerant) archaeon, is capable of natural genetic transformation. Cytoplasmic bridges are formed between cells that appear to be used for DNA transfer from one cell to another in either direction.[39]
Sulfolobus solfataricus and Sulfolobus acidocaldarius are hyperthermophilic archaea. Exposure of these organisms to the DNA damaging agents UV irradiation, bleomycin or mitomycin C induces species-specific cellular aggregation.[40][41] UV-induced cellular aggregation of S. acidocaldarius mediates chromosomal marker exchange with high frequency.[41] Recombination rates exceed those of uninduced cultures by up to three orders of magnitude. Frols et al.[40] and Ajon et al.[41] hypothesized that cellular aggregation enhances species-specific DNA transfer between Sulfolobus cells in order to repair damaged DNA by means of homologous recombination. Van Wolferen et al.[42] noted that this DNA exchange process may be crucial under DNA damaging conditions such as high temperatures. It has also been suggested that DNA transfer in Sulfolobus may be an early form of sexual interaction similar to the more well-studied bacterial transformation systems that involve species-specific DNA transfer leading to homologous recombinational repair of DNA damage[43] (and see Transformation (genetics)).
Extracellular membrane vesicles (MVs) might be involved in DNA transfer between different hyperthermophilic archaeal species.[44] It has been shown that both plasmids[45] and viral genomes[44] can be transferred via MVs. Notably, a horizontal plasmid transfer has been documented between hyperthermophilic Thermococcus and Methanocaldococcus species, respectively belonging to the orders Thermococcales and Methanococcales.[46]
See also
References
- ↑ Rampelotto, P. H. (2010). "Resistance of microorganisms to extreme environmental conditions and its contribution to Astrobiology". Sustainability. 2 (6): 1602–1623. Bibcode:2010Sust....2.1602R. doi:10.3390/su2061602.
- ↑ Rothschild, L.J.; Mancinelli, R.L. (2001). "Life in extreme environments". Nature. 409 (6823): 1092–1101. Bibcode:2001Natur.409.1092R. doi:10.1038/35059215. PMID 11234023.
- ↑ "Mars Exploration Rover Launches - Press kit" (PDF). NASA. June 2003. Retrieved 14 July 2009.
- ↑ BBC Staff (23 August 2011). "Impacts 'more likely' to have spread life from Earth". BBC. Retrieved 24 August 2011.
- ↑ Gorman, James (6 February 2013). "Bacteria Found Deep Under Antarctic Ice, Scientists Say". The New York Times. Retrieved 6 February 2013.
- 1 2 3 Choi, Charles Q. (17 March 2013). "Microbes Thrive in Deepest Spot on Earth". LiveScience. Retrieved 17 March 2013.
- ↑ Glud, Ronnie; Wenzhöfer, Frank; Middleboe, Mathias; Oguri, Kazumasa; Turnewitsch, Robert; Canfield, Donald E.; Kitazato, Hiroshi (17 March 2013). "High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth". Nature Geoscience. 6 (4): 284–288. Bibcode:2013NatGe...6..284G. doi:10.1038/ngeo1773. Retrieved 17 March 2013.
- ↑ Oskin, Becky (14 March 2013). "Intraterrestrials: Life Thrives in Ocean Floor". LiveScience. Retrieved 17 March 2013.
- 1 2 3 4 Reed, Christopher J.; Lewis, Hunter; Trejo, Eric; Winston, Vern; Evilia, Caryn (2013). "Protein Adaptations in Archaeal Extremophiles". Archaea. 2013: 1–14. doi:10.1155/2013/373275. PMC 3787623. PMID 24151449.
- ↑ "NASA Astrobiology Strategy" (PDF). NASA. 2015. p. 59.
- ↑ Marteinsson, VT; Birrien, JL; Reysenbach, AL; Vernet, M; Marie, D; Gambacorta, A; Messner, P; Sleytr, UB; Prieur, D (1999). "Thermococcus barophilus sp. nov., a new barophilic and hyperthermophilic archaeon isolated under high hydrostatic pressure from a deep-sea hydrothermal vent". International Journal of Systematic and Evolutionary Microbiology. 49 (2): 351–359. doi:10.1099/00207713-49-2-351. PMID 10319455.
- ↑ Yadav, Ajar Nath; Verma, Priyanka; Kumar, Murugan; Pal, Kamal K.; Dey, Rinku; Gupta, Alka; Padaria, Jasdeep Chatrath; Gujar, Govind T.; Kumar, Sudheer (2014-05-31). "Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India". Annals of Microbiology. 65 (2): 611–629. doi:10.1007/s13213-014-0897-9. ISSN 1590-4261.
- ↑ Cavicchioli, R. & Thomas, T. 2000. Extremophiles. In: J. Lederberg. (ed.) Encyclopedia of Microbiology, Second Edition, Vol. 2, pp. 317–337. Academic Press, San Diego.
- ↑ "Studies refute arsenic bug claim". BBC News. 9 July 2012. Retrieved 10 July 2012.
- ↑ Erb, Tobias J.; Kiefer, Patrick; Hattendorf, Bodo; Günther, Detlef; Vorholt, Julia A. (8 July 2012). "GFAJ-1 Is an Arsenate-Resistant, Phosphate-Dependent Organism". Science. 337 (6093): 467–70. Bibcode:2012Sci...337..467E. doi:10.1126/science.1218455. PMID 22773139. Retrieved 10 July 2012.
- ↑ Reaves, Marshall Louis; Sinha, Sunita; Rabinowitz, Joshua D.; Kruglyak, Leonid; Redfield, Rosemary J. (8 July 2012). "Absence of Detectable Arsenate in DNA from Arsenate-Grown GFAJ-1 Cells". Science. 337 (6093): 470–3. arXiv:1201.6643. Bibcode:2012Sci...337..470R. doi:10.1126/science.1219861. PMC 3845625. PMID 22773140. Retrieved 10 July 2012.
- ↑ Dworkin, Martin; Falkow, Stanley (13 July 2006). The Prokaryotes: Vol. 1: Symbiotic Associations, Biotechnology, Applied Microbiology. Springer. p. 94. ISBN 978-0-387-25476-0.
- ↑ Ward, P. D.; Brownlee, D. (2004). The life and death of planet Earth. New York: Owl Books. ISBN 978-0-8050-7512-0.
- ↑ Chang, Kenneth (12 September 2016). "Visions of Life on Mars in Earth's Depths". The New York Times. Retrieved 12 September 2016.
- ↑ Wynn-Williams, D. A.; Newton, E. M.; Edwards, H. G. (2001). Exo-/astro-biology : proceedings of the first European workshop, 21 - 23 May 2001, ESRIN, Fracscati, Italy. Exo-/astro-Biology. 496. p. 226. Bibcode:2001ESASP.496..225W. ISBN 978-92-9092-806-5.
- ↑ Than, Ker (25 April 2011). "Bacteria Grow Under 400,000 Times Earth's Gravity". National Geographic- Daily News. National Geographic Society. Retrieved 28 April 2011.
- ↑ Deguchi, Shigeru; Hirokazu Shimoshige, Mikiko Tsudome, Sada-atsu Mukai, Robert W. Corkery, Susumu Ito, and Koki Horikoshi; Tsudome, M.; Mukai, S.-a.; Corkery, R. W.; Ito, S.; Horikoshi, K. (2011). "Microbial growth at hyperaccelerations up to 403,627 xg". Proceedings of the National Academy of Sciences. 108 (19): 7997–8002. Bibcode:2011PNAS..108.7997D. doi:10.1073/pnas.1018027108. PMC 3093466. PMID 21518884. Retrieved 28 April 2011.
- ↑ Baldwin, Emily (26 April 2012). "Lichen survives harsh Mars environment". Skymania News. Retrieved 27 April 2012.
- ↑ de Vera, J.-P.; Kohler, Ulrich (26 April 2012). "The adaptation potential of extremophiles to Martian surface conditions and its implication for the habitability of Mars" (PDF). European Geosciences Union. Retrieved 27 April 2012.
- ↑ Kim W; et al. (29 April 2013). "Spaceflight Promotes Biofilm Formation by Pseudomonas aeruginosa". PLoS ONE. 8 (4): e6237. Bibcode:2013PLoSO...862437K. doi:10.1371/journal.pone.0062437. PMC 3639165. PMID 23658630.
- ↑ Madhusoodanan, Jyoti (19 May 2014). "Microbial stowaways to Mars identified". Nature. doi:10.1038/nature.2014.15249. Retrieved 23 May 2014.
- ↑ Fox, Douglas (20 August 2014). "Lakes under the ice: Antarctica's secret garden". Nature. 512 (7514): 244–246. Bibcode:2014Natur.512..244F. doi:10.1038/512244a. PMID 25143097. Retrieved 21 August 2014.
- ↑ Mack, Eric (20 August 2014). "Life Confirmed Under Antarctic Ice; Is Space Next?". Forbes. Retrieved 21 August 2014.
- ↑ Mastascusa, V.; Romano, I.; Di Donato, P.; Poli, A.; Della Corte, V.; Rotundi, A.; Bussoletti, E.; Quarto, M.; Pugliese, M.; Nicolaus, B. (9 January 2015). "Extremophiles Survival to Simulated Space Conditions: An Astrobiology Model Study". Origins of Life and Evolution of Biospheres. 44 (3): 231–237. Bibcode:2014OLEB...44..231M. doi:10.1007/s11084-014-9397-y. PMC 4669584. PMID 25573749.
- ↑ Barney, Brandon L.; Pratt, Sara N.; Austin, Daniel E. (June 2016). "Survivability of bare, individual Bacillus subtilis spores to high-velocity surface impact: Implications for microbial transfer through space". Planetary and Space Science. 125: 20–26. Bibcode:2016P&SS..125...20B. doi:10.1016/j.pss.2016.02.010.
- ↑ Microbial Life Found in Hydrocarbon Lake. the physics arXiv blog 15 April 2010.
- ↑ Schulze-Makuch, Haque, Antonio, Ali, Hosein, Song, Yang, Zaikova, Beckles, Guinan, Lehto, Hallam. Microbial Life in a Liquid Asphalt Desert.
- ↑ Ahmed, Iftikhar; Yokota, Akira; Fujiwara, Toru (2006). "A novel highly boron tolerant bacterium, Bacillus boroniphilus sp. nov., isolated from soil, that requires boron for its growth". Extremophiles. 11 (2): 217–224. doi:10.1007/s00792-006-0027-0. PMID 17072687.
- ↑ "Bioenergy and Industrial Microbiology". Idaho National Laboratory. U.S. Department of Energy. Retrieved 3 February 2014.
- ↑ Anitori, RP (editor) (2012). Extremophiles: Microbiology and Biotechnology. Caister Academic Press. ISBN 978-1-904455-98-1.
- ↑ Johnsborg, O; Eldholm, V; Håvarstein, LS. (2007). "Natural genetic transformation: prevalence, mechanisms and function". Res Microbiol. 158 (10): 767–78. doi:10.1016/j.resmic.2007.09.004. PMID 17997281.
- ↑ Moseley, BE; Setlow, JK. (1968). "Transformation in Micrococcus radiodurans and the ultraviolet sensitivity of its transforming DNA". Proc Natl Acad Sci U S A. 61 (1): 176–83. Bibcode:1968PNAS...61..176M. doi:10.1073/pnas.61.1.176. PMC 285920. PMID 5303325.
- ↑ Koyama, Y; Hoshino, T; Tomizuka, N; Furukawa, K. (1986). "Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp". J Bacteriol. 166 (1): 338–40. PMC 214599. PMID 3957870.
- ↑ Rosenshine, I; Tchelet, R; Mevarech, M. (1989). "The mechanism of DNA transfer in the mating system of an archaebacterium". Science. 245 (4924): 1387–9. Bibcode:1989Sci...245.1387R. doi:10.1126/science.2818746. PMID 2818746.
- 1 2 Fröls, S; Ajon, M; Wagner, M; Teichmann, D; Zolghadr, B; Folea, M; Boekema, EJ; Driessen, AJ; Schleper, C; et al. (2008). "UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation". Mol Microbiol. 70 (4): 938–52. doi:10.1111/j.1365-2958.2008.06459.x. PMID 18990182.
- 1 2 3 Ajon, M; Fröls, S; van Wolferen, M; Stoecker, K; Teichmann, D; Driessen, AJ; Grogan, DW; Albers, SV; Schleper, C.; et al. (2011). "UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili". Mol Microbiol. 82 (4): 807–17. doi:10.1111/j.1365-2958.2011.07861.x. PMID 21999488.
- ↑ Van Wolferen, M; Ajon, M; Driessen, AJ; Albers, SV. (2013). "How hyperthermophiles adapt to change their lives: DNA exchange in extreme conditions". Extremophiles. 17 (4): 545–63. doi:10.1007/s00792-013-0552-6. PMID 23712907.
- ↑ Bernstein H and Bernstein C (2013). Evolutionary Origin and Adaptive Function of Meiosis, Meiosis, Dr. Carol Bernstein (Ed.), ISBN 978-953-51-1197-9, InTech, http://www.intechopen.com/books/meiosis/evolutionary-origin-and-adaptive-function-of-meiosis
- ↑ Gaudin M, Gauliard E, Schouten S, Houel-Renault L, Lenormand P, Marguet E, Forterre P.; Gauliard; Schouten; Houel-Renault; Lenormand; Marguet; Forterre (2013). "Hyperthermophilic archaea produce membrane vesicles that can transfer DNA". Environ Microbiol Rep. 5 (1): 109–16. doi:10.1111/j.1758-2229.2012.00348.x. PMID 23757139.
- ↑ Krupovic M, Gonnet M, Hania WB, Forterre P, Erauso G; Gonnet; Hania; Forterre; Erauso (2013). "Insights into dynamics of mobile genetic elements in hyperthermophilic environments from five new Thermococcus plasmids". PLoS ONE. 8 (1): e49044. Bibcode:2013PLoSO...849044K. doi:10.1371/journal.pone.0049044. PMC 3543421. PMID 23326305.
Further reading
- Wilson, Z. E.; Brimble, M. A. (January 2009). "Molecules derived from the extremes of life". Nat. Prod. Rep. 26 (1): 44–71. doi:10.1039/b800164m. PMID 19374122.
- Rossi M; et al. (July 2003). "Extremophiles 2002". J. Bacteriol. 185 (13): 3683–9. doi:10.1128/JB.185.13.3683-3689.2003. PMC 161588. PMID 12813059.
- C.Michael Hogan (2010). "Extremophile". Encyclopedia of Earth, National Council of Science & the Environment, Eds. E,Monosson & C.Cleveland.
- Joseph Seckbach, et al.: Polyextremophiles: life under multiple forms of stress. Springer, Dordrecht 2013, ISBN 978-94-007-6488-0.
External links
| Wikinews has related news: Bacteria thrive deep under sea floor |
- Extreme Environments - Science Education Resource Center
- Extremophile Research
- Eukaryotes in extreme environments
- The Research Center of Extremophiles
- DaveDarling's Encyclopedia of Astrobiology, Astronomy, and Spaceflight
- The International Society for Extremophiles
- Idaho National Laboratory
- Polyextremophile on David Darling's Encyclopedia of Astrobiology, Astronomy, and Spaceflight
- T-Limit Expedition