Cleanroom




A cleanroom or clean room is a facility ordinarily utilized as a part of specialized industrial production or scientific research, including the manufacture of pharmaceutical items and microprocessors. Cleanrooms are designed to maintain extremely low levels of particulates, such as dust, airborne organisms, or vaporized particles. Cleanrooms typically have an cleanliness level quanitified by the number of particles per cubic meter at a predetermined molecule measure. The ambient outdoor air in a typical urban area contains 35,000,000 particles for each cubic meter in the size range 0.5 μm and bigger in measurement, equivalent to an ISO 9 cleanroom, while by comparison an ISO 1 cleanroom permits no particles in that size range and just 12 particles for each cubic meter of 0.3 μm and smaller.
History
The modern cleanroom was invented by American physicist Willis Whitfield.[1] As employee of the Sandia National Laboratories, Whitfield created the initial plans for the cleanroom in 1960.[1] Prior to Whitfield's invention, earlier cleanrooms often had problems with particles and unpredictable airflows. Whitfield designed his cleanroom with a constant, highly filtered air flow to flush out impurities.[1] Within a few years of its invention in the 1960s, Whitfield's modern cleanroom had generated more than 50 billion USD in sales worldwide.[2][3]
The majority of the integrated circuit manufacturing facilities in Silicon Valley were made by three companies: MicroAire, PureAire, and Key Plastics. These competitors made laminar flow units, glove boxes, clean rooms and air showers, along with the chemical tanks and benches used in the 'Wet Process' building of integrated circuits. These three companies were the pioneers of the use of Teflon for airguns, chemical pumps, scrubbers, water guns, and other devices needed for the production of [[integrated circuit]s. William (Bill) C. McElroy Jr. worked as engineering manager, drafting room supervisor, QA/QC, and designer for all three companies and his designs added 45 original patents to the technology of the time. McElroy also wrote a four page article for MicroContamination Journal, wet processing training manuals, and equipment manuals for wet processing and clean rooms.[4]
Overview
Cleanrooms can be very large. Entire manufacturing facilities can be contained within a cleanroom with factory floors covering thousands of square meters. They are used extensively in semiconductor manufacturing, biotechnology, the life sciences, and other fields that are very sensitive to environmental contamination. There are also modular cleanrooms.[5]
The air entering a cleanroom from outside is filtered to exclude dust, and the air inside is constantly recirculated through high-efficiency particulate air (HEPA) and/or ultra-low particulate air (ULPA) filters to remove internally generated contaminants.
Staff enter and leave through airlocks (sometimes including an air shower stage), and wear protective clothing such as hoods, face masks, gloves, boots, and coveralls.
Equipment inside the cleanroom is designed to generate minimal air contamination. Only special mops and buckets are used. Cleanroom furniture is designed to produce a minimum of particles and is easy to clean.
Common materials such as paper, pencils, and fabrics made from natural fibers are often excluded, and alternatives used. Cleanrooms are not sterile (i.e., free of uncontrolled microbes);[6] only airborne particles are controlled. Particle levels are usually tested using a particle counter and microorganisms detected and counted through environmental monitoring methods.[7][8]
Some cleanrooms are kept at a positive pressure so if any leaks occur, air leaks out of the chamber instead of unfiltered air coming in.
Some cleanroom HVAC systems control the humidity to such low levels that extra equipment like air ionizers are required to prevent electrostatic discharge problems.
Low-level cleanrooms may only require special shoes, with completely smooth soles that do not track in dust or dirt. However, for safety reasons, shoe soles must not create slipping hazards. Access to a cleanroom is usually restricted to those wearing a cleanroom suit.
In cleanrooms in which the standards of air contamination are less rigorous, the entrance to the cleanroom may not have an air shower. An anteroom (known as a "gray room") is used to put on clean-room clothing.
Some manufacturing facilities do not use fully classified cleanrooms, but use some practices or technologies typical of cleanrooms to meet their contamination requirements.
In Hospitals, Theatres are similar to cleanroom for surgical patients' operation with incisions to prevent any infections for the patient.
Air flow principles
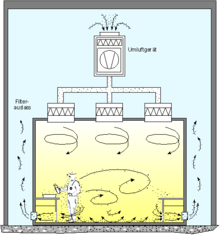 Air flow pattern for "Turbulent Cleanroom" |
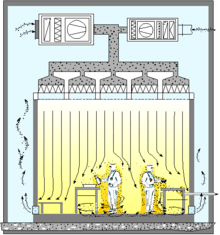 Air flow pattern for "Laminar Flow Cleanroom" |
Cleanrooms maintain particulate-free air through the use of either HEPA or ULPA filters employing laminar or turbulent air flow principles. Laminar, or unidirectional, air flow systems direct filtered air downward or in horizontal direction in a constant stream towards filters located on walls near the cleanroom floor or through raised perforated floor panels to be recirculated. Laminar air flow systems are typically employed across 80% of a cleanroom ceiling to maintain constant air processing. Stainless steel or other non shedding materials are used to construct laminar air flow filters and hoods to prevent excess particles entering the air. Turbulent, or non unidirectional, air flow uses both laminar air flow hoods and nonspecific velocity filters to keep air in a cleanroom in constant motion, although not all in the same direction. The rough air seeks to trap particles that may be in the air and drive them towards the floor, where they enter filters and leave the cleanroom environment. US FDA and EU have laid down guidelines and limit for microbial contamination which is very stringent to ensure freedom from microbial contamination in pharmaceutical products.[9][10]
Personnel contamination of cleanrooms
In the healthcare and pharmaceutical sectors, control of microorganisms is important, especially microorganisms likely to be deposited into the air stream from skin shedding. Studying cleanroom microflora is of importance for microbiologists and quality control personnel to assess changes in trends. Shifts in the types of microflora may indicate deviations from the “norm” such as resistant strains or problems with cleaning practices.
In assessing cleanroom microorganisms, the typical flora are primarily those associated with human skin (Gram-positive cocci), although microorganisms from other sources such as the environment (Gram-positive rods) and water (Gram-negative rods) are also detected, although in lower number. Common bacterial genera include Micrococcus, Staphylococcus, Corynebacterium, and Bacillus, and fungal genera include Aspergillus and Pencillin.[8]
Cleanroom classification and standardization
Cleanrooms are classified according to the number and size of particles permitted per volume of air. Large numbers like "class 100" or "class 1000" refer to FED-STD-209E, and denote the number of particles of size 0.5 µm or larger permitted per cubic foot of air. The standard also allows interpolation; for example SNOLAB is maintained as a class 2000 cleanroom.
A discrete, light-scattering airborne particle counter is used to determine the concentration of airborne particles, equal to and larger than the specified sizes, at designated sampling locations.
Small numbers refer to ISO 14644-1 standards, which specify the decimal logarithm of the number of particles 0.1 µm or larger permitted per m3 of air. So, for example, an ISO class 5 cleanroom has at most 105 particles/m3.
Both FS 209E and ISO 14644-1 assume log-log relationships between particle size and particle concentration. For that reason, zero particle concentration does not exist. Some classes do not require testing some particle sizes, because the concentration is too low or too high to be practical to test for, but such blanks should not be read as zero.
Because 1 m3 is about 35 ft3, the two standards are mostly equivalent when measuring 0.5 µm particles, although the testing standards differ. Ordinary room air is around class 1,000,000 or ISO 9.[11]
ISO 14644-1 and ISO 14698
ISO 14644-1 and ISO 14698 are non-governmental standards developed by the International Organization for Standardization (ISO).[12] The former applies to clean rooms in general (see table below); the latter to cleanrooms where biocontamination may be an issue.
| Class | Maximum particles/m3 a | FED STD 209E equivalent | |||||
|---|---|---|---|---|---|---|---|
| ≥0.1 µm | ≥0.2 µm | ≥0.3 µm | ≥0.5 µm | ≥1 µm | ≥5 µm | ||
| ISO 1 | 10b | d | d | d | d | e | |
| ISO 2 | 100 | 24b | 10b | d | d | e | |
| ISO 3 | 1,000 | 237 | 102 | 35b | d | e | Class 1 |
| ISO 4 | 10,000 | 2,370 | 1,020 | 352 | 83b | e | Class 10 |
| ISO 5 | 100,000 | 23,700 | 10,200 | 3,520 | 832 | d,e,f | Class 100 |
| ISO 6 | 1,000,000 | 237,000 | 102,000 | 35,200 | 8,320 | 293 | Class 1,000 |
| ISO 7 | c | c | c | 352,000 | 83,200 | 2,930 | Class 10,000 |
| ISO 8 | c | c | c | 3,520,000 | 832,000 | 29,300 | Class 100,000 |
| ISO 9 | c | c | c | 35,200,000 | 8,320,000 | 293,000 | Room air |
| a All concentrations in the table are cumulative, e.g. for ISO Class 5, the 10 200 particles shown at 0,3 μm include all particles equal to and greater than this size. b These concentrations will lead to large air sample volumes for classification. Sequential sampling procedure may be applied; see Annex D. | |||||||
US FED STD 209E
US FED-STD-209E was a United States federal standard. It was officially cancelled by the General Services Administration on November 29, 2001,[13][14] but is still widely used.[15]
| Class | Maximum particles/ft3 | ISO equivalent | ||||
|---|---|---|---|---|---|---|
| ≥0.1 µm | ≥0.2 µm | ≥0.3 µm | ≥0.5 µm | ≥5 µm | ||
| 1 | 35 | 7.5 | 3 | 1 | 0.007 | ISO 3 |
| 10 | 350 | 75 | 30 | 10 | 0.07 | ISO 4 |
| 100 | 3,500 | 750 | 300 | 100 | 0.7 | ISO 5 |
| 1,000 | 35,000 | 7,500 | 3000 | 1,000 | 7 | ISO 6 |
| 10,000 | 350,000 | 75,000 | 30,000 | 10,000 | 70 | ISO 7 |
| 100,000 | 3.5×106 | 750,000 | 300,000 | 100,000 | 830 | ISO 8 |
EU GMP classification
EU GMP guidelines are more stringent than others, requiring cleanrooms to meet particle counts at operation (during manufacturing process) and at rest (when manufacturing process is not carried out, but room AHU is on).
| Class | Maximum particles/m3[16] | |||
|---|---|---|---|---|
| At Rest | In Operation | |||
| 0.5 µm | 5 µm | 0.5 µm | 5 µm | |
| Grade A | 3,520 | 20 | 3,520 | 20 |
| Grade B | 3,520 | 29 | 352,000 | 2,900 |
| Grade C | 352,000 | 2,900 | 3,520,000 | 29,000 |
| Grade D | 3,520,000 | 29,000 | Not defined | Not defined |
BS 5295
BS 5295 is a British Standard.
| Class | Maximum particles/m3 | |||||
|---|---|---|---|---|---|---|
| ≥0.5 µm | ≥1 µm | ≥5 µm | ≥10 µm | ≥25 µm | ||
| Class 1 | 3,000 | 0 | 0 | 0 | ||
| Class 2 | 300,000 | 2,000 | 30 | |||
| Class 3 | 1,000,000 | 20,000 | 4,000 | 300 | ||
| Class 4 | 200,000 | 40,000 | 4,000 | |||
BS 5295 Class 1 also requires that the greatest particle present in any sample can not exceed 5 μm.[17] BS 5295 has been superseded, withdrawn since the year 2007 and replaced with "BS EN ISO 14644-6:2007".[18]
See also
References
- 1 2 3 Yardley, William (2012-12-04). "Willis Whitfield, Clean Room Inventor, Dies at 92". The New York Times. Retrieved 2013-06-22.
- ↑ "Sandia physicist, cleanroom inventor dies at 92". KWES. Associated Press. 2012-11-26. Retrieved 2012-12-03.
- ↑ "Willis Whitfield - Father of the Cleanroom" (PDF). Cleanroom online. September 2015. Retrieved 2016-05-18.
- ↑ William (Bill) C. McElroy Jr., MicroAire Engineering Manager and acting VP; Kay Plastics Engineering Manager; PureAire Drafting Room Manager
- ↑ What is a modular cleanroom?
- ↑ In NASA’s Sterile Areas, Plenty of Robust Bacteria New York Times, 9. October 2007
- ↑ Sandle, T (November 2012). "Application of quality risk management to set viable environmental monitoring frequencies in biotechnology processing and support areas". PDA J Pharm Sci Technol. 66 (6): 560–79. doi:10.5731/pdajpst.2012.00891.
- 1 2 Sandle, T (November 2011). "A review of cleanroom microflora: types, trends, and patterns". PDA J Pharm Sci Technol. 65 (4): 392–403. doi:10.5731/pdajpst.2011.00765.
- ↑ Limits for Microbial load for clean room as per US FDA and EU Guidelines for pharmaceutical products
- ↑ Cleanroom Air Flow Principles
- ↑ Cleanroom Classification / Particle Count / FS209E / ISO TC209 /
- ↑ "ISO 14644-1:2015 - Cleanrooms and associated controlled environments -- Part 1: Classification of air cleanliness by particle concentration". ISO. Retrieved 2016-09-12.
- ↑ Cancellation of FED-STD-209E - Institute of Environmental Sciences and Technology
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2008-05-28. Retrieved 2008-04-17. , page 148
- ↑ "NUFAB SAFETY & PROTOCOL" (PDF). Retrieved 24 February 2016.
- ↑ Understanding Cleanroom Classifications
- ↑ Market Venture Philippines Inc. web site (2006-04-19). "What is a Clean Room?". Archived from the original on 2012-08-28. Retrieved 2007-06-02.
- ↑ "BS 5295-0:1989 - Environmental cleanliness in enclosed spaces. General introduction, terms and definitions for clean rooms and clean air devices". 2016. Retrieved 2016-04-18.
External links
| Wikimedia Commons has media related to Cleanrooms. |