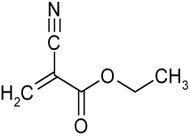
Ethyl cyanoacrylate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
ethyl 2-cyanopropenoate | |
| Other names
ethyl 2-cyanoacrylate, ECA, ethyl alpha-cyanoacrylate, 910EM, ace-ee, CN2, CN4, cemedine 3000rs, Krazy glue, permabond 105, permabond 200, super glue, pro grip 4000, TK 200, TK 201, cyanolite 201, Cyanacrine, Cyano-Veneer | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.027.628 |
| UNII | |
| |
| |
| Properties | |
| C6H7NO2 | |
| Molar mass | 125.13 g/mol |
| Density | 1.06 g/mL |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 54 to 56 °C (129 to 133 °F; 327 to 329 K) at 3mm Hg |
| Hazards | |
| Flash point | 83 °C (181 °F; 356 K) |
| 0.2 ppm | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethyl cyanoacrylate (ECA), a cyanoacrylate ester, is an ethyl ester of 2-cyano-2-propenoic acid. It is a colorless liquid with low viscosity and a faint sweet smell in pure form. It is the main component of cyanoacrylate glues and can be encountered under many trade names. "Super glue" is believed to be ECA. The makers of "Krazy Glue" state on their website, "The chemical name for Krazy Glue is ethyl cyanoacrylate."[1] Mercury Adhesives is an example of an American-made ECA.[2] It is soluble in acetone, methyl ethyl ketone, nitromethane, and methylene chloride.[3] ECA polymerizes rapidly in presence of moisture.
Production
Ethyl cyanoacrylate is prepared by the condensation of formaldehyde with ethyl cyanoacetate:
This exothermic reaction affords the polymer, which is subsequently sintered, thermally "cracked" to give the monomer. Alternatively, it can be prepared by the ethoxycarbonylation of cyanoacetylene.[4]
Applications
Ethyl cyanoacrylate is used for gluing various materials. It is also used in medicine, for liquid bandages and for suture-less surgery, but it is used less often than the less toxic n-butyl and octyl cyanoacrylates. Off-the-shelf non-medical-grade glues are unsuitable for medical applications, as they may contain solvents (e.g. methyl alcohol) and produce heat during polymerization.[5]
After curing, the resulting resin softens at temperatures above 150 °C (302 °F). The service temperature of the joint is −54–82 °C (−65–180 °F). Its dielectric constant at 1 megahertz is 3.33.[6]
Safety
In the U.S., the Threshold Limit Value for ECA is 0.2 ppm. Heating causes depolymerization of the cured poly-ECA, producing gaseous products which are a strong irritant to the lungs and eyes.
See also
References
- ↑ http://www.krazyglue.com/about-us
- ↑ http://www.krazyglue.com/products/msds/mkg0583_a.htm
- ↑ https://web.archive.org/web/20090603175358/http://palmlabsadhesives.com/technical_data.htm
- ↑ Takashi Ohara, Takahisa Sato, Noboru Shimizu, Günter Prescher, Helmut Schwind, Otto Weiberg, Klaus Marten, Helmut Greim (2002). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH,. doi:10.1002/14356007.a01_161.pub2.
- ↑ http://www.miracleglue.com/wounds.htm
- ↑ "Archived copy". Archived from the original on 2008-12-08. Retrieved 2008-12-08.