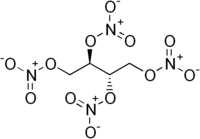
Erythritol tetranitrate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
[(2R,3R)-1,3,4-Trinitrooxybutan-2-yl] nitrate | |
| Other names
Erythrityl tetranitrate (INN) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.027.940 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C4H6N4O12 | |
| Molar mass | 302.11 g·mol−1 |
| Density | 1.7219 (±0.0025) g/cm3 |
| Melting point | 61 °C (142 °F; 334 K) |
| Boiling point | Decomposes at 160 °C |
| Hazards | |
| GHS pictograms |    |
| NFPA 704 | |
| Explosive data | |
| Shock sensitivity | Medium (2.0 Nm) |
| Friction sensitivity | Medium |
| Detonation velocity | 8000-8100 m/s |
| RE factor | 1.60 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Erythritol tetranitrate (ETN) is an explosive compound chemically similar to PETN.[1] It is however thought to be 1/3 more sensitive to friction and impact. ETN is not well known, but in recent years has been used by amateur experimenters to replace PETN in improvised detonation cord or in boosters to initiate larger, less sensitive explosive charges. Due to the availability of erythritol as a natural sweetener and its relative ease of production in relation to PETN, ETN is a favoured homemade explosive compound to the amateur experimenter.
Like many nitrate esters, ETN acts as a vasodilator, and was the active ingredient in the original "sustained release" tablets, made under a process patent in the early 1950s, called "nitroglyn". Ingesting ETN or prolonged skin contact can lead to absorption and what is known as a "nitro headache".
Properties
ETN has a relatively high velocity of detonation of 8000–8100 m/s at a density of 1.7219 (±0.0025) g/cm3.[2] It is white in color and odorless. ETN is commonly cast into mixtures with other high explosives. It is somewhat sensitive to shock and friction, so care should be taken while handling. ETN dissolves readily in acetone and other ketone solvents. The impact and friction sensitivity is slightly higher than the sensitivity of PETN. The sensitivity of melt cast and pressed ETN is comparable. Lower nitrates of erythritol, like erythritol trinitrate are soluble in water, so they do not contaminate most ETN samples.
Much like PETN, ETN is known for having a very long shelf life. Studies that directly observed the crystalline structure saw no signs of decomposition after four years of storage at room temperature. ETN has a melting point of 61 °C, compared to PETN which has a melting point of 141.3 °C. Recent studies of ETN decomposition suggested a unimolecular rate-limiting step in which the O-NO2 bond is cleaved and begins the decomposition sequence.[3]
ETN can and should be recrystalized, as to remove the trapped acids from synthesis. Warm ethanol or methanol is a viable solvent (close to 10 g of ETN/100 ml EtOH). ETN will precitipate as big platelets with bulk density of about 0.3 g/cm3 (fluffy material) when crashed into several liters of cold water. Smaller, fine crystals are produced by slow addition of water in said ETN/ethanol solution with intense mixing. ETN can be easily hand pressed to about 1.2 g/cm3 (with a slight risk of accidental detonation).
ETN can be melt-casted in warm (~65°C) water. Slight decomposition is possible (often betrayed by change in color from white to very light yellow). Nonetheless, no reports of runaway reactions leading to explosion have been confirmed (when melt casting using only a bucket of warm water and recrystalized ETN). Melt cast ETN, if cooled down slowly over a period of 10 - 30 minutes, has density of 1.70 g/cm3, detonation velocity of 8040 m/s and Pcj detonation pressure of about 300 kbar. Its brisance is far higher than that of Semtex (about 220 kbar, depending on brand)[4][5][6]
ETN is often plasticized using PIB/synthethic oil binders (very comparable to the binder system in C4) or using liquid nitric esters. The PIB based plastic explosives are non-toxic and completely comparable to C4 or Semtex with Pcj between 200 and 250 kbar, depending on density (influenced by crystal size, binder amount and amount of final rolling). EGDN/ETN/NC systems are toxic to touch, quite sensitive to friction and impact but generally slightly more powerful than C4 (Pcj of about 250 kbar na Edet = 5.3 MJ/Kg) and more powerful than Semtex (Pcj of about 220 kbar and Edet = below 5 MJ/kg)- with Pcj of about 250 - 270 kbar and Edet around 6 MJ/kg. Note that different explosive softwares and different experimental tests will yield absolute detonation pressures that can vary by 5 % or more with the relative proportions being maintained.
Oxygen balance
One quality this explosive has, that PETN does not, is a positive oxygen balance. Having a positive oxygen balance means that ETN possesses more than enough oxygen in its structure to fully oxidize all of its carbon and hydrogen upon detonation. This can be seen in the schematic chemical equation below.
- 2 C4H6N4O12 → 8 CO2 + 6 H2O + 4 N2 + 1 O2
Whereas PETN decomposes to:
- 2 C5H8N4O12 → 6 CO2 + 8 H2O + 4 N2 + 4 CO
The carbon monoxide (CO) still requires oxygen to complete oxidation to carbon dioxide (CO2). A detailed study of the decomposition chemistry of ETN has been recently elucidated.[3]
Thus for every two moles of ETN that decomposes, one free mole of O2 is released. This could be used to oxidize an added metal dust or an oxygen deficient explosive such as TNT or PETN.
Manufacture
Like other nitrated polyols, ETN is made by nitrating erythritol through the mixing of concentrated sulfuric acid and a nitrate salt. Thoroughly dried Ammonium nitrate or potassium nitrate is commonly used for this type of reaction. Ammonium nitrate is superior in terms of yields and ease of manufacture. The erythritol is added to the mixture to begin its nitration. Much better yields can be obtained by using concentrated nitric acid in place of the nitrate salt, in which case the sulfuric acid is used simply to absorb water and act as a catalyst from the resulting esterification, driving the reaction. The first part of the reaction requires significant cooling in an ice bath, the KNO3/sulfuric acid route needs about an hour of cooling, conc. HNO3/sulfuric acid route requires about 25 minutes. It is important to perform the last step at about 20°C - for about an hour in the case of KNO3/sulfuric route or for about 5 minutes in the case of concentrated nitric acid/sulfuric acid mix. Lower final temperature is going to yield only the trinitrate and lower esters and these are soluble in water. The reaction times for ammonium nitrate route are intermediate between these two. Using a closed, glass container with a plastic lid is a common amateur practice, that limits the amount of escaping NOx fumes. Constant mixing via shaking, glass rod or a magnetic stirrer is required.
See also
References
- ↑ Erythritol tetranitrate was first synthesized by British chemist John Stenhouse (1809-1880) in 1849. He extracted the simple sugar erythritol (which he called "erythroglucin") from lichen and then studied its chemistry. See: John Stenhouse (1 January 1849) "Examination of the proximate principles of some of the lichens. Part II," Philosophical Transactions of the Royal Society (London), vol. 139, pages 393-401. Reprinted in German as: John von Stenhouse (1849) "Über die näheren Bestandtheile einige Flechten," Justus Liebigs Annalen der Chemie und Pharmacie, vol. 70, no. 2, pages 218-228. Condensed version (in German): John Stenhouse (12 Sept. 1849) "Über die näheren Bestandtheile einige Flechten," Pharmaceutisches Centralblatt, vol. 20, no. 40, pages 625-628.
- ↑ http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.653.6239&rep=rep1&type=pdf
- 1 2 Furman, David; Kosloff, Ronnie; Zeiri, Yehuda (2016-12-22). "Effects of Nanoscale Heterogeneities on the Reactivity of Shocked Erythritol Tetranitrate". The Journal of Physical Chemistry C. 120 (50): 28886–28893. doi:10.1021/acs.jpcc.6b11543. ISSN 1932-7447.
- ↑ Oxley, Jimmie C.; Smith, James L.; Brady, Joseph E.; Brown, Austin C. (2012-02). "Characterization and Analysis of Tetranitrate Esters". Propellants, Explosives, Pyrotechnics. 37 (1): 24–39. doi:10.1002/prep.201100059. ISSN 0721-3115. Check date values in:
|date=(help) - ↑ Künzel, Martin; Matyas, Robert; Vodochodský, Ondřej; Pachman, Jiri (2017-05-04). "Explosive Properties of Melt Cast Erythritol Tetranitrate (ETN)" (PDF). Central European Journal of Energetic Materials. 14 (2): 418–429. doi:10.22211/cejem/68471. ISSN 1733-7178.
- ↑ Oxley, Jimmie C.; Furman, David; Brown, Austin C.; Dubnikova, Faina; Smith, James L.; Kosloff, Ronnie; Zeiri, Yehuda (2017-07-18). "Thermal Decomposition of Erythritol Tetranitrate: A Joint Experimental and Computational Study". The Journal of Physical Chemistry C. 121 (30): 16145–16157. doi:10.1021/acs.jpcc.7b04668. ISSN 1932-7447.
