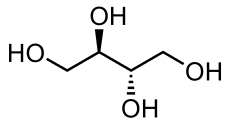
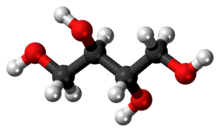
Erythritol
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
(2R,3S)-Butane-1,2,3,4-tetrol | |
| Other names
(2R,3S)-Butane-1,2,3,4-tetraol (not recommended) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.217 |
| E number | E968 (glazing agents, ...) |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C4H10O4 | |
| Molar mass | 122.12 g·mol−1 |
| Density | 1.45 g/cm3 |
| Melting point | 121 °C (250 °F; 394 K) |
| Boiling point | 329 to 331 °C (624 to 628 °F; 602 to 604 K) |
| −73.80·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Erythritol ((2R,3S)-butane-1,2,3,4-tetrol) is a sugar alcohol (or polyol) that has been approved for use as a food additive in the United States[1] and throughout much of the world. It was discovered in 1848 by Scottish chemist John Stenhouse.[2] It occurs naturally in some fruit and fermented foods.[3] At the industrial level, it is produced from glucose by fermentation with a yeast, Moniliella pollinis.[1] Erythritol is 60–70% as sweet as sucrose (table sugar) yet it is almost noncaloric,[4] does not affect blood sugar,[5] does not cause tooth decay,[6] and is partially absorbed by the body, excreted in urine and feces. Under U.S. Food and Drug Administration (FDA) labeling requirements, it has a caloric value of 0.2 kilocalories per gram (95% less than sugar and other carbohydrates), though nutritional labeling varies from country to country. Some countries, such as Japan and the United States, label it as zero-calorie; the European Union labels it 0 kcal/g.[7]
Human digestion
In the body, most erythritol is absorbed into the bloodstream in the small intestine, and then for the most part excreted unchanged in the urine. About 10% enters the colon.[8] Because 90% of erythritol is absorbed before it enters the large intestine, it does not normally cause laxative effects, as are often experienced after consumption of other sugar alcohols (such as xylitol and maltitol),[9] although large doses can cause nausea and stomach rumbling.[10] In males, doses greater than 0.66 g/kg body weight and in females, doses greater than 0.8 g/kg body weight, will cause laxation.[11]
Side effects
In general, there are no known side effects for erythritol in regular use. Doses over 50 grams (1.8 oz) can cause a significant increase in nausea and stomach rumbling.[10] Rarely, erythritol can cause allergic hives (urticaria).[12]
When compared with other sugar alcohols, it is also much more difficult for intestinal bacteria to digest, so it is less likely to cause gas or bloating than other polyols,[8] such as maltitol, sorbitol, or lactitol.
According to a 2014 study,[13] erythritol functions as an insecticide toxic to the fruit fly Drosophila melanogaster, impairing motor ability and reducing longevity even when nutritive sugars were available.
Production
Erythritol is produced industrially beginning with enzymatic hydrolysis of the starch from corn to generate glucose.[14] Glucose is then fermented with yeast or another fungus to produce erythritol. Other methods such as electrochemical synthesis are in development.[15]
Physical properties
Heat of solution
Erythritol has a strong cooling effect (endothermic, or positive heat of solution)[16] when it dissolves in water, which is often compared with the cooling effect of mint flavors. The cooling effect is present only when erythritol is not already dissolved in water, a situation that might be experienced in an erythritol-sweetened frosting, chocolate bar, chewing gum, or hard candy. The cooling effect of erythritol is very similar to that of xylitol and among the strongest cooling effects of all sugar alcohols.[17]
Blending for sugar-like properties
Erythritol is commonly used as a medium in which to deliver high-intensity sweeteners, especially stevia derivatives, serving the dual function of providing both bulk and a flavor similar to that of table sugar. Diet beverages are made with this blend, thus contain erythritol in addition to the main sweetener. Beyond high-intensity sweeteners, erythritol is often paired with other bulky ingredients that exhibit sugar-like characteristics to better mimic the texture and mouthfeel of sucrose. The cooling effect of erythritol is rarely desired, hence other ingredients are chosen to dilute or negate that effect. Erythritol also has a propensity to crystallize and is not as soluble as sucrose, so ingredients may also be chosen to help negate this disadvantage. Furthermore, erythritol is not hygroscopic, meaning it does not attract moisture, which can lead to the drying out of products, in particular baked goods, if another hygroscopic ingredient is not used in the formulation.
Inulin is often combined with erythritol because of inulin's offering a complementary negative heat of solution (exothermic, or warming effect when dissolved, which helps cancel erythritol's cooling effect) and noncrystallizing properties. However, inulin has a propensity to cause gas and bloating in those having consumed it in moderate to large quantities, in particular in individuals unaccustomed to it. Other sugar alcohols are sometimes used with erythritol, in particular isomalt, because of its minimally positive heat of solution, and glycerin, which has a negative heat of solution, moderate hygroscopicity, and noncrystallizing liquid form.
Bacteria
Erythritol is tooth-friendly; it cannot be metabolized by oral bacteria, so it does not contribute to tooth decay.[6]
Erythritol is preferentially utilized by the Brucella bacteria spp. The presence of erythritol in the placentas of goats, cows, and pigs has been proposed as an explanation for the accumulation of Brucella bacteria found at these sites.[18]
Synonyms
In the 19th and early 20th centuries, several synonyms were in use for erythritol: erythrol, erythrite, erythoglucin, eryglucin, erythromannite and phycite.[19]
See also
- Erythritol tetranitrate
- Pentaerythritol
- Stevia
- Threitol, the diastereomer of erythritol
References
- 1 2 FDA/CFSAN: Agency Response Letter: GRAS Notice No. GRN 000076
- ↑ The discovery of erythritol, which Stenhouse called "erythroglucin", was announced in: Stenhouse, J. (January 1, 1848). "Examination of the proximate principles of some of the lichens". Philosophical Transactions of the Royal Society of London. 138: 63–89, see especially p. 76. doi:10.1098/rstl.1848.0004.
- ↑ Shindou, T.; Sasaki, Y.; Miki, H.; Eguchi, T.; Hagiwara, K.; Ichikawa, T. (1988). "Determination of erythritol in fermented foods by high performance liquid chromatography" (pdf). Shokuhin Eiseigaku Zasshi. 29 (6): 419–22. doi:10.3358/shokueishi.29.419.
- ↑ Vasudevan, D. M. (2013). Textbook of biochemistry for medical students. New Delhi: Jaypee Brothers Medical Publishers (P) LTD. p. 81. ISBN 978-93-5090-530-2.
- ↑ Moon, HJ; Jeya, M; Kim, IW; Lee, JK (April 2010). "Biotechnological production of erythritol and its applications". Applied Microbiology and Biotechnology. 86 (4): 1017–25. doi:10.1007/s00253-010-2496-4. PMID 20186409.
- 1 2 Kawanabe, J.; Hirasawa, M.; Takeuchi, T.; Oda, T.; Ikeda, T. (1992). "Noncariogenicity of erythritol as a substrate". Caries Research. 26 (5): 358–62. doi:10.1159/000261468. PMID 1468100.
- ↑
European Commission Directive 2008/100/EC changed the energy conversion values of erythritol to zero calories:
- Erythritol is a polyol, and according to the current rules as provided for in Article 5(1) of Directive 90/496/EEC, its energy would be calculated using the conversion factor for polyols, namely 10 kJ/g (2,4 kcal/g). Using this energy conversion factor would not fully inform the consumer about the reduced energy value of a product achieved by the use of erythritol in its manufacture. The Scientific Committee on Food in its opinion on erythritol, expressed on March 5, 2003, noted that the energy provided by erythritol was less than 0,9 kJ/g (less than 0,2 kcal/g). Therefore it is appropriate to adopt a suitable energy conversion factor for erythritol. Current regulations (Reg. (EC) 1169/2011) preserve this conversion factor at 0 kcal/g for energy value calculation purposes.
- 1 2 Arrigoni, E.; Brouns, F.; Amadò, R. (November 2005). "Human gut microbiota does not ferment erythritol" (pdf). British Journal of Nutrition. 94 (5): 643–6. doi:10.1079/BJN20051546. PMID 16277764.
- ↑ Munro, I. C.; Berndt, W. O.; Borzelleca, J. F.; et al. (December 1998). "Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data". Food and Chemical Toxicology. 36 (12): 1139–74. doi:10.1016/S0278-6915(98)00091-X. PMID 9862657.
- 1 2 Storey, D.; Lee, A.; Bornet, F.; Brouns, F. (Mar 2007). "Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid". European Journal of Clinical Nutrition. 61 (3): 349–54. doi:10.1038/sj.ejcn.1602532. PMID 16988647.
- ↑ Mäkinen KK (2016). "Gastrointestinal Disturbances Associated with the Consumption of Sugar Alcohols with Special Consideration of Xylitol: Scientific Review and Instructions for Dentists and Other Health-Care Professionals". Int J Dent. 2016: 5967907. doi:10.1155/2016/5967907. PMC 5093271. PMID 27840639.
- ↑ Hino, H.; Kasai, S.; Hattori, N.; Kenjo, K. (Mar 2000). "A case of allergic urticaria caused by erythritol". Journal of Dermatology. 27 (3): 163–5. PMID 10774141.
- ↑ Baudier, K.M.; Kaschock-Marenda, S.D.; Patel, N.; Diangelus, K.L.; O'Donnell, S.; Marenda, D.R. (2014). "Erythritol, a Non-Nutritive Sugar Alcohol Sweetener and the Main Component of Truvia®, Is a Palatable Ingested Insecticide". PLoS ONE. 9 (6): e98949. doi:10.1371/journal.pone.0098949.
- ↑ Clara Piccirillo, PhD (January 28, 2014). "How Is Erythritol Made? Manufacture of a Low-Calorie Sugar Substitute". Decoded Science.
- ↑ Elaine Watson (April 10, 2013). "'Green electrochemistry' could pave way for more cost effective production of erythritol, says trailblazing DFI Corp".
- ↑ Wohlfarth, C. (2006). CRC handbook of enthalpy data of polymer-solvent systems. CRC / Taylor & Francis. pp. 3–. ISBN 978-0-8493-9361-7.
- ↑ Jasra, R. V.; Ahluwalia, J. C. (1982). "Enthalpies of Solution, Partial Molal Heat Capacities and Apparent Molal Volumes of Sugars and Polyols in Water". Journal of Solution Chemistry. 11 (5): 325–338. doi:10.1007/BF00649291. ISSN 1572-8927.
- ↑ Petersen, Erik; Rajashekara, Gireesh; Sanakkayala, Neelima; Eskra, Linda; Harms, Jerome; Splitter, Gary (2013). "Erythritol triggers expression of virulence traits in Brucella melitensis". Microbes and Infection. 15 (6–7): 440–449. doi:10.1016/j.micinf.2013.02.002. ISSN 1286-4579. PMC 3686989.
- ↑ "A list of words whose use should be avoided in favor of the accompanying synonyms". Journal of Anaytical and Applied Chemistry. 6: 160. 1892.
External links

