Epimorphosis
Epimorphosis is defined as the regeneration of a specific part of an organism in a way that involves extensive cell proliferation,[1] as well as blastema formation.[2] Epimorphosis can be considered a simple model for development, though it only occurs in tissues surrounding the site of injury rather than occurring system-wide.[3]
History of Epimorphosis
Thomas Hunt Morgan, an evolutionary biologist who also worked with embryology, argued that limb and tissue reformation bore many similarities to embryonic development.[4] Building off of the work of German embryologist Wilhelm Roux, who suggested regeneration was two cooperative but distinct pathways instead of one, Morgan named the two parts of the regenerative process epimorphosis and morphallaxis. Specifically, Morgan wanted epimorphosis to specify the process of entirely new tissues being regrown from an amputation or similar injury, with morphallaxis being coined to describe regeneration that did not use cell proliferation, such as in hydra[5]
Epimorphosis in vertebrates
_(21260217591).jpg)
In vertebrates, epimorphosis relies on blastema formation to proliferate cells into the new tissue. Through studies involving zebrafish fins, the toetips of mice, and limb regeneration in axolotls, researchers at the Polish Academy of Sciences found evidence for epimorphosis occurring in a variety of vertebrates, including instances of mammal epimorphosis.[8]
Salamanders
Epidermal cells at the wound margins migrate to cover the wound and will become the wound epidermis.[9] No scar tissue forms, as it would in mammals.
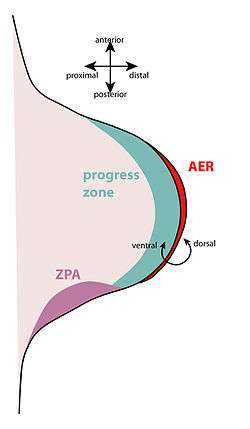
An apical ectodermal cap (AEC) forms on the tip of the stump.[10] This is similar to the embryonic apical ectodermal ridge, which forms during normal limb development. The AEC causes the progress zone to re-establish; this means the cells under the AEC dedifferentiate and become mesenchymal. The AEC releases fibroblast growth factors (FGFs) (including FGF-4 and -8) that drive the development of the new limb, essentially resetting the limb back to its embryonic development stage.[11]
However, even though some of the limb cells are able to dedifferentiate, they are not able to fully dedifferentiate to the level of multipotent progenitor cells. During regeneration, only cartilage cells can form new cartilage tissue, only muscle cells can form new muscle tissue, and so on. The dedifferentiated cells still retain their original specification.[9]
Epimorphosis in invertebrates
Periplaneta americana
The American cockroach is capable of regenerating limbs that have been damaged or destroyed, such as legs and antennae, as well parts of its compound eye. It does this with lectin—a protein made for binding proteins—named regenectin, which shares a family with other lipopolysaccharide (LPS) binding proteins. Regenectin carries both a regenerative and a system defense function, and it is produced by the cockroach's paracrine system to work with muscle reformation.[3]
Capitella teleta
C. teleta is a segmented worm found in North America that is capable of regenerating posterior segments after amputation.[12] This regeneration uses the interaction of several sets of Hox genes, as well as blastema formation. All of the Hox genes concerned in epimorphosis are present in the abdominal area of the worm, but not in the anterior portion. However, the genes do not, themselves, direct the anterior-posterior patterning of the worm's thorax.[13]
Planaria vitta
P. vitta is a flatworm of genus Planaria that, when needed, can draw upon both morphallaxis and epimorphosis to regrow itself; in P. vitta, epimorphosis precedes morphallaxis and lasts about ten days. The epidermis contracts immediately after the worm is cut at the head, which activates totipotent regenerative cells, and rhabdites gather on the epidermis. Near the time the eye-spots on the worm begin to take form, epimorphosis begins to shift over to morphallaxis, which then finishes regrowth into a complete individual.[14]
References
- ↑ "Medical Definition of EPIMORPHOSIS". www.merriam-webster.com. Retrieved 2018-02-19.
- ↑ Yokoyama H (January 2008). "Initiation of limb regeneration: the critical steps for regenerative capacity". Development, Growth & Differentiation. 50 (1): 13–22. doi:10.1111/j.1440-169X.2007.00973.x. PMID 17986260.
- 1 2 Kubo T, Arai T (September 1996). "Insect Lectins and Epimorphosis". Trends in Glycoscience and Glycotechnology. Japanese Society of Carbohydrate Research. 8 (43): 357–364.
- ↑ Sunderland ME (2010-05-01). "Regeneration: Thomas Hunt Morgan's window into development". Journal of the History of Biology. 43 (2): 325–61. doi:10.1007/s10739-009-9203-2. PMID 20665231.
- ↑ "Thomas Hunt Morgan's Definition of Regeneration: Morphallaxis and Epimorphosis". The Embryo Project Encyclopedia. Retrieved 2018-02-19.
- ↑ Summerbell D, Lewis JH, Wolpert L (August 1973). "Positional information in chick limb morphogenesis". Nature. 244 (5417): 492–6. doi:10.1038/244492a0. PMID 4621272.
- ↑ Huxley J, De Beer G (1963). The Elements of Experimental Embryology. New York: Hafner Pub. Co.
- ↑ Conn PM. Animal models for the study of human disease (Second ed.). London, United Kingdom. ISBN 978-0-12-809699-4. OCLC 992170104.
- 1 2 Gilbert SF (2014). Developmental Biology (Tenth ed.). Sunderland, MA, USA: Sinauer Associates, Inc. pp. 571–573.
- ↑ Issues in Biological, Biochemical, and Evolutionary Sciences Research. Atlanta, GA: ScholarlyEditions. 2012. p. 464.
- ↑ Nye, Holly L.D.; Cameron, Jo Ann; Chernoff, Ellen A.G.; Stocum, David L. "Regeneration of the urodele limb: A review". Developmental Dynamics. 226 (2): 280–294. doi:10.1002/dvdy.10236.
- ↑ Fröbius, Andreas C.; Matus, David Q.; Seaver, Elaine C. (2008-12-23). "Genomic Organization and Expression Demonstrate Spatial and Temporal Hox Gene Colinearity in the Lophotrochozoan Capitella sp. I". PLOS One. 3 (12): e4004. doi:10.1371/journal.pone.0004004. ISSN 1932-6203.
- ↑ de Jong DM, Seaver EC (2016-02-19). "A Stable Thoracic Hox Code and Epimorphosis Characterize Posterior Regeneration in Capitella teleta". PLOS One. 11 (2): e0149724. doi:10.1371/journal.pone.0149724. PMC 4764619. PMID 26894631.
- ↑ Pedersen, K. J. (1958). "Morphogenetic Activities during Planarian Regeneration as influenced by Triethylene Melamine". Development. The Company of Biologists. 6 (2): 308–334. eISSN 1477-9129.