Dodecylbenzene
| Names | |
|---|---|
| IUPAC name
Dodecylbenzene | |
| Other names
1-Phenyldodecane, Phenyldodecane, n-Dodecylbenzene, Laurylbenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.175 |
| |
| |
| Properties | |
| C18H30 | |
| Molar mass | 246.43 g/mol |
| Appearance | colourless liquid |
| Density | 0.856 g/cm3 |
| Melting point | −7 °C (19 °F; 266 K) |
| Boiling point | 290 to 410 °C (554 to 770 °F; 563 to 683 K) (mixture of isomers) |
| insoluble | |
| Hazards | |
| NFPA 704 | |
| Flash point | 135 °C (275 °F; 408 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dodecylbenzene is an organic compound with the formula C12H25C6H5.Dodecylbenzene is a colorless liquid with a weak oily odor. Floats on water.
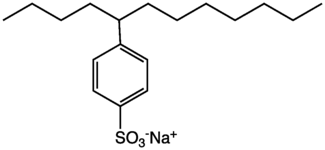
This colourless waxy solid consists of a dodecyl group (C12H25) attached to a phenyl group (C6H5). Dodecylbenzene is a precursor to sodium dodecylbenzenesulfonate, a surfactant that is a key ingredient of household laundry detergents, such as detergent powder.[1]
Production
This compound and some related alkylbenzene are produced industrially by alkylation of benzene with the corresponding alkenes in the presence of hydrogen fluoride or related acid catalysts. The resulting linear alkylbenzene compounds are sulfonated to give the corresponding sulfonic acids. This sulfonation can be highly specific to place the sulfonic acid group across the ring, in the 4-position. The resulting sulfonic acid is then neutralized with base to give sodium alkylbenzenesulfonate, which is subsequently blended with other components to give various cleaning products.[1]
References
- 1 2 Kurt Kosswig,"Surfactants" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, Weinheim. doi:10.1002/14356007.a25_747
- ↑ Bipin V. Vora, Joseph A. Kocal, Paul T. Barger, Robert J. Schmidt, James A. Johnson (2003). "Alkylation". Kirk‐Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0112112508011313.a01.pub2.
