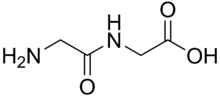
Dipeptide
A dipeptide is an organic compound derived from two amino acids. The amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Similar descriptions apply to tripeptide (three amino acid residues, two peptide bonds), tetrapeptide, and so on; longer chains are called oligopeptide, polypeptide, protein.
Production
Synthetic dipeptides
Dipeptides are produced by coupling amino acids. The amino group on one amino acid is rendered non-nucleophilic (P in eq) and the carboxylic acid group in the second amino acid is deactivated as its methyl ester. The two modified amino acids are then combined in the presence of a coupling agent, which facilitates formation of the amide bond:
- RCH(NHP)CO2H + R'CH(NH2)CO2CH3 → RCH(NHP)C(O)NH(CHR')CO2CH3 + H2O
Subsequent to this coupling reaction, the amine protecting group P and the ester are converted to the free amine and carboxylic acid, respectively.[1]
For many amino acids, the ancillary functional groups are protected. The condensation of the amine and the carboxylic acid to form the peptide bond generally employs coupling agents to activate the carboxylic acid.[2]
The Bergmann azlactone peptide synthesis is a classic organic synthesis for the preparation of dipeptides.[3]
Biosynthesis
Dipeptides are produced from polypeptides by the action of the hydrolase enzyme dipeptidyl peptidase.[4] Dietary proteins are digested to dipeptides and amino acids, and the dipeptides are absorbed more rapidly than the amino acids, because their uptake involves a separate mechanism. Dipeptides activate G-cells found in the stomach to secrete gastrin.
Examples
- Carnosine (beta-alanyl-L-histidine) is highly concentrated in muscle and brain tissues.
- Anserine (beta-alanyl-N-methyl histidine) is found in the skeletal muscle and brain of mammals.
- Homoanserine (N-(4-aminobutyryl)-L-histidine) is another dipeptide identified in the brain and muscles of mammals.
- Kyotorphin (L-tyrosyl-L-arginine) is a neuroactive dipeptide which plays a role in pain regulation in the brain.
- Balenine (or ophidine) (beta-alanyl-N tau-methyl histidine) has been identified in the muscles of several species of mammal (including man), and the chicken.
- Aspartame (N-L-α-aspartyl-L-phenylalanine 1-methyl ester) is an artificial sweetener.
- Glorin (N-propionyl-γ-L-glutamyl-L-ornithine-δ-lac ethyl ester) is a chemotactic dipeptide for the slime mold Polysphondylium violaceum.
- Barettin (cyclo-[(6-bromo-8-en-tryptophan)-arginine]) is a cyclic dipeptide from the marine sponge Geodia barretti.
- Pseudoproline
- Glycylglycine
One amino acid, two peptide bonds
In this usage, X dipeptide is taken literally: One amino acid X is equipped with two minimal peptide bonds: The C terminus COOH becomes COCH3, the N terminus NH2 becomes NHCH3. For instance, alanine dipeptide is CH3CONHCH(CH3)CONHCH3.[5]
References
- ↑ Subirós-Funosas, Ayman El-Faham, Fernando Albericio (2013). "Low-epimerization Peptide Bond Formation with Oxyma Pure: Preparation of Z-L-Phg-Val-OMe". Org. Synth. 90: 306. doi:10.15227/orgsyn.090.0306.
- ↑ Jean-Simon Suppo, Renata Marcia de Figueiredo, Jean-Marc Campagne (2015). "Dipeptide Syntheses via Activated α-Aminoesters". Org. Synth. 92: 296. doi:10.15227/orgsyn.092.0296.
- ↑ Bergmann, M. et al., Ann. 449, 277 (1926); "Bergmann Azlactone Peptide Synthesis"
- ↑ Steane, Richard. "Hydrolysis of a dipeptide". BioTopics. Retrieved 28 July 2014.
- ↑ Head-Gordon, Teresa; Head-Gordon, Martin; Frisch, Michael J.; Brooks, Charles; Pople, John (2009). "A theoretical study of alanine dipeptide and analogs". International Journal of Quantum Chemistry. 36: 311. doi:10.1002/qua.560360725.
External links
- An introduction to dipeptides at PeptideGuide.