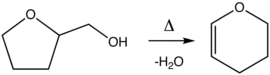Dihydropyran
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
3,4-Dihydro-2H-pyran 3,6-dihydro-2H-pyran | |||
| Other names
2,3-Dihydro-4H-pyran | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.003.465 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C5H8O | |||
| Molar mass | 84.12 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.922 g/mL | ||
| Melting point | −70 °C (−94 °F; 203 K) | ||
| Boiling point | 86 °C (187 °F; 359 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
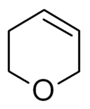
Dihydropyran is a heterocyclic compound with the formula C5H8O. The six-membered, non-aromatic ring has five carbon atoms and one oxygen atom. It contains one double bond. There are two isomers of dihydropyran that differ by the location of the double bond. 3,4-Dihydro-2H-pyran has a double bond at position 5; 3,6-dihydro-2H-pyran has the double bond at position 4.
Nomenclature
In IUPAC names, "dihydro" refers to the two added hydrogen atoms needed to remove one double bond from the parent compound pyran. The numbers in front of the prefix indicate the position of the added hydrogen atoms.[1] The italicized capital H denotes the "indicated hydrogen", that is a second hydrogen atom present on the location where no double bond is present. The indicated hydrogen is placed just in front of the parent hydride (pyran).[2]
Note that the position numbers of the ring-members not follow the position of the double bonds here. If also the second double bond is removed by two more hydrogen atoms we get tetrahydropyran, or oxane.
3,4-Dihydropyran
3,4-Dihydropyran, also known as 2,3-dihydropyran, is used for protecting several chemicals.[3]
Preparation
Dihydropyran is prepared by the dehydration of tetrahydrofurfuryl alcohol (THFA) over alumina at 300–400 °C.[4] THFA is itself prepared from tetrahydro-2-furoic acid.
Reactions
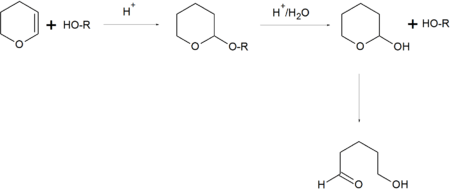
In organic synthesis, the 2-tetrahydropyranyl group is used as a protecting group for alcohols.[5][6] Reaction of the alcohol with 3,4-dihydropyran forms a tetrahydropyranyl ether, protecting the alcohol from a variety of reactions. The alcohol can later be restored readily by acidic hydrolysis with formation of 5-hydroxypentanal.[7]
See also
References
- ↑ A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993): R-3.1.2 Hydro prefixes
- ↑ A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993): R-1.3 Indicated Hydrogen
- ↑ Technical information from Penn Chemicals
- ↑ R. L. Sawyer and D. W. Andrus (1955). "2,3-Dihydropyran". Organic Syntheses. ; Collective Volume, 3, p. 276
- ↑ R. A. Earl L. B. Townsend (1990). "Methyl 4-Hydroxy-2-butynoate". Organic Syntheses. ; Collective Volume, 7, p. 334
- ↑ Arthur F. Kluge (1990). "Diethyl [(2-Tetrahydropyranyloxy)methyl]phosphonate". Organic Syntheses. ; Collective Volume, 7, p. 160
- ↑ Wuts, Peter G. M.; Greene, Theodora W. (2006). "Protection for the Hydroxyl Group, Including 1,2‐ and 1,3‐Diols". Greene's Protective Groups in Organic Synthesis (4th ed.). pp. 16–366. doi:10.1002/9780470053485.ch2. ISBN 9780470053485.