Degradation of MCPA in soil
MCPA, 4-chloro-2-methylphenoxyacetic acid, is included in the group of phenoxy herbicides. MCPA has been extensively used in agriculture to control broad-leaf weeds as a growth regulator primarily in pasture and cereal crops field since 1945. The mode of action of MCPA appears to be similar to auxins, which are growth hormone that naturally exist in plants. Overdose application of MCPA acts as an herbicide and results abnormal growth.[1][2]

Behaviors in soil
MCPA herbicide is usually sprayed to soil surface and plant leaves surface in its water solution,sometimes with additional surfactant. MCPA in soil can be absorbed by plants root, and translocated in phloem to leaves and stems. The MCPA residue left in soil typically have a half life of 24 days.[3] However, the degradation rate is depending on environmental conditions, such as temperature and soil moisture.[4] MCPA is rather mobile in soil, and not very adsorpted to soil particles, with Kf = 0.94 and 1/n = 0.68 of Freundlich adsorption.[3][4]
Environmental Risks
Widely usage of MCPA as an herbicide rises concern of environmental risks, a lot researches have been done in recent decades to evaluate the environmental risk of MCPA. MCPA can be moderately toxic to mammal and aquatic organisms, and relatively lower toxic to birds.[5] MCP (4-chloro-2-methylphenol) is the intermediate in the synthesis of phenoxy herbicide, and is also the metabolite of MCPA's degradation. It has been estimated as a total of 15000 tons of MCP were produced in 1989 in EU.[6] The MCP chemical is considered very toxic to aquatic organisms. However, the concentration of MCPA and MCP detected in water and soil are lower than the predicted no effect levels of all environmental compartments, and considered under low potential of risk.[6][7]
The carboxyl group of MCPA can form conjugated complex with metals as a ligand.[8] In general pH range of aqueous environment,the MCPA-metal complex has higher solubility than metal ion. MCPA may be environmentally hazardous by affecting the mobility and bio-availability of heavy metal, such as cadmium and lead.The acid functionality makes MCPA a versatile synthetic intermediate for more complex derivatives[9]
-COOH + M+ → -COOM + H+
Bio-degradation
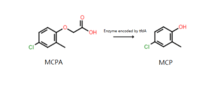
The MCPA can be degraded biologically in soils by plants and microorganisms. The major metabolite of MCPA degradation are MCP (4-chloro-2-methylphenol). The pathway could be the cleavage of the ether linkage, yielding MCP and acetate acid. Another pathway could be the hydroxylation of methyl group, yielding cloxyfonac (4-Chloro-2-hydroxymethylphenoxyacetic acid). Recent studies demonstrated that biologically degradation of MCPA is enzymatically catalyzed by an α-ketoglutarate-dependent dioxygenase encoded by the tfdA gene of soil microorganisms. Soil indigenous bacteria that carry tfdA gene could use MCPA as the sole source of carbon.[10][11]
Photo-degradation
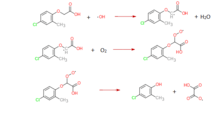
MCPA also could be photochemical degraded. Two scheme pathways can be proposed for the formation of the main intermediate MCP. One scheme is MCPA oxidation by hydroxyl radical, •OH. Hydroxyl radical adds on the ring, then the radical transfer to ether carbon. With oxygen present, the addition of hydroxyl radical leads the cleavage of ether link, yielding MCP. The other scheme is MCPA oxidation by positive electron holes h+. The positive holes h+ polarize carboxyl group, CH2-COOH bond is split to produce 4-chloro-2-methylphenylformate. With the presence of oxygen, the positive holes h+ oxidation finally yields MCP as well.[9]

Summary
Since MCPA is extensive used in USA, the extensively dispensed MCPA and its biological and photochemical metabolites might be environmentally hazardous. However, current studies showed that there is no resistance of MCPA to degrade in soil.
References
- ↑ Reade, J., Cobb, A. H. (2002). Herbicides: Modes of action and metabolism. Weed management handbook. pp. 157–158.
- ↑ Grossmann, K. (2010). "Auxin herbicides: current status of mechanism and mode of action". Pest Management Science. 66: 2033–2043.
- 1 2 University of Hertfordshire (2016-10-17). "MCPA". sitem.herts.ac.uk. Retrieved 2016-11-21.
- 1 2 Helweg, A. (1987). "Degradation and adsorption of 14C-MCPA in soil—influence of concentration, temperature and moisture content on degradation". Weed Research. 27: 287–296.
- ↑ "Ecotoxicology of MCPA". Pesticide Properties DataBase. University of Hertfordshire. Retrieved 2016-10-17.
- 1 2 UNEP publications, OECD SIDS, 4-chloro-2-methylphenol. http://www.inchem.org/documents/sids/sids/1570645.pdf
- ↑ Guidelines for drinking-water quality, 2nd ed. Vol.2. Health criteria and other supporting information. World Health Organization, Geneva, 1996.
- ↑ Bala, Tanushree; Prasad, B. L. V.; Sastry, Murali; Kahaly, Mousumi Upadhyay; Waghmare, Umesh V. (2007-07-19). "Interaction of different metal ions with carboxylic acid group: a quantitative study". The Journal of Physical Chemistry A. 111 (28): 6183–6190. doi:10.1021/jp067906x. ISSN 1089-5639. PMID 17585841.
- 1 2 Zertal, A.; Molnár-Gábor, D.; Malouki, M.A.; Sehili, T.; Boule, P. (2004-05-01). "Photocatalytic transformation of 4-chloro-2-methylphenoxyacetic acid (MCPA) on several kinds of TiO2". Applied Catalysis B: Environmental. 49 (2): 83–89. doi:10.1016/j.apcatb.2003.11.015. ISSN 0926-3373.
- ↑ Bælum, Jacob; Nicolaisen, Mette H.; Holben, William E.; Strobel, Bjarne W.; Sørensen, Jan; Jacobsen, Carsten S. (2008-03-20). "Direct analysis of tfdA gene expression by indigenous bacteria in phenoxy acid amended agricultural soil". The ISME Journal. 2 (6): 677–687. doi:10.1038/ismej.2008.21. ISSN 1751-7362.
- ↑ Nielsen, Morten S.; Bælum, Jacob; Jensen, Malene B.; Jacobsen, Carsten S. (2011-05-01). "Mineralization of the herbicide MCPA in urban soils is linked to presence and growth of class III tfdA genes". Soil Biology and Biochemistry. 43 (5): 984–990. doi:10.1016/j.soilbio.2011.01.014.