Criegee oxidation
The Criegee oxidation is a glycol cleavage reaction in which vicinal diols are oxidized to form ketones and aldehydes using lead tetraacetate. It is analogous but milder than the Malaprade reaction. This oxidation was discovered by Rudolf Criegee and coworkers and first reported in 1931; their seminal publication investigated the oxidative cleavage of glycol.[1]

The rate of the reaction is highly dependent on the conformation of the diols, so much so that diols that are cis on certain rings can be reacted selectively as opposed to those that are trans on them.[2] It was heavily stressed by Criegee that the reaction must be run in anhydrous solvents, as any water present would hydrolyze the lead tetraacetate; however, subsequent publications have shown that if the oxidation is faster than the rate of hydrolysis, the cleavage can be run in wet solvents or even aqueous solutions.[3] For example, gluose, glycerol, mannitol, and xylose can all undergo a Criegee oxidation in aqueous solutions, but sucrose cannot.[4][5]
Mechanism
There are two mechanisms given for the Criegee oxidation which depend on the configuration of the diol.[6] If the two hydroxy groups are conformationally close to each other, a five-membered ring can be formed with the lead atom as a linker between the two oxygen atoms. If the structure cannot adopt a conformation that has the hydroxy groups close enough to form this ring, an alternate mechanism is possible, but is slower.[7] Trans-fused five member rings are heavily strained, thus trans-diols that are on a five-membered ring will react slower than cis-alcohols on such a structure.[8]
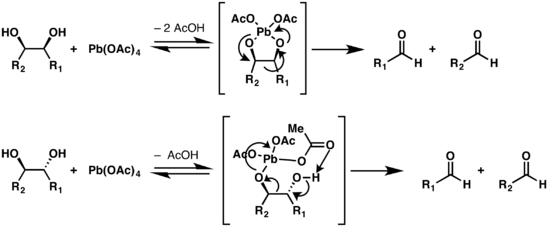
Modifications
Although the classic substrate for the Criegee oxidation are 1,2-diols, the oxidation can be employed with α-amino alcohols,[9] α-hydroxy carbonyls,[10] and α-keto acids,[11] In the case of α-amino alcohols, a free radical mechanism is proposed.
A cascade Criegee ring expansion reaction has been shown on bicylic cyclohex-3-ene-1,2-diols. The product of the oxidative cleavage of the diol, a dialdehyde, underdoes a reversible hetero-Diels–Alder reaction, whose product is captured by lead tetraacetate. An ensuing migration of the bond between two of the rings and pericyclic reaction of the lead acetate portion results in the ring being expanded by one carbon atom. This process can yield either 6- or 7- member rings by starting with the appropriate 5- or 6-membered ring precursor.[12][13]
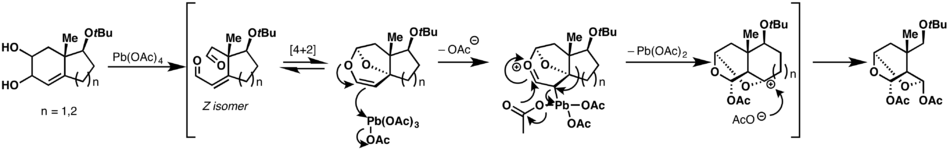
The Crigee oxidation can also be employed with epoxides to form α-acetoxy carbonyls. This technique, in tandem with directed epoxidation can yield chiral molecules.[14]

Synthetic applications
Criegee oxidations are commonly used in carbohydrate chemistry to cleave 1,2-glycols and differentiate between different kinds of glycol groups.[15] The following reactions are highlighting the diversity of the Criegee oxidation in chemical synthesis.[8]
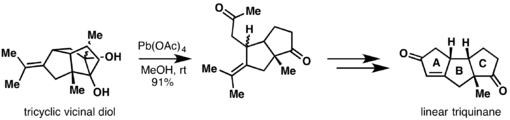
Rao and coworkers completed the synthesis of linear and angular triquinanes through a 5-exo-trig allyl radical cylcization as their key step. The A ring was installed by Criegee oxidation of their tricyclic vicinal diol to a diketone, which upon ozonolysis, could undergo a Robinson annulation to form their final compound.[16]
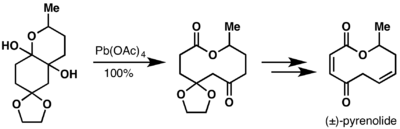
M. Hesse and coworkers utilized the Criegee oxidation as their key ring expansion step in the total synthesis of (±)-pyrenolide B.[17]
References
- ↑ Crieege, R. (1931). "Eine oxydative Spaltung von Glykolen". Berichte der deutschen chemischen Gesellschaft. 64: 260–266.
- ↑ Reeves, R. E. (1949). "Direct Titration of cis-Glycols with Lead Tetraacetate. Analytical Chemistry". 21: 751–751.
- ↑ Baer, Grosheintz, Fischer, E.; J. M.; H. O. L.; (1939). "Oxidation of 1,2-Glycols or 1,2,3-Polyalcohols by Means of Lead Tetraacetate in Aqueous Solution". Journal of the American Chemical Society. 61: 2607–2609. doi:10.1021/ja01265a010.
- ↑ Hockett, Zief, M., R. C.; M. (1950). "Lead Tetraacetate Oxidations in the Sugar Group. XI.1 The Oxidation of Sucrose and Preparation of Glycerol and Glycol". Journal of the American Chemical Society. 72: 2130–2132.
- ↑ Abraham, S. (1950). "The Quantitative Recovery of Carbon Dioxide in Lead Tetraacetate Oxidations of Sugars and Sugar Derivatives". Journal of the American Chemical Society. 72: 4050–4053.
- ↑ Criegee, Büchner, Walther, R.; E.; W. (1940). "Die Geschwindigkeit der Glykolspaltung mit BleiIV-acetat in Abhängigkeit von der Konstitution des Glykols". Berichte der deutschen chemischen Gesellschaft. 73: 571–575.
- ↑ Mihailović, Čeković, Mathes,, M. L.; Z., B. M. (2005). "Lead(IV) Acetate". e-EROS Encyclopedia of Reagents for Organic Synthesis.
- 1 2 László, Barbara, Kürti, Czakó (2005). Strategic Applications of Named Reactions in Organic Synthesis. Murlington, MA: Elsevier Academic Press. pp. 114–115. ISBN 978-0-12-429785-2.
- ↑ Leonard, Rebenstorf, N. J.; M. A. (1945). "Lead Tetraacetate Oxidation of Aminoalcohols". Journal of the American Chemical Society. 67: 49–51. doi:10.1021/ja01217a016.
- ↑ Evans, Bender, Morris,, D. A.; S. L.; J. (1988). "The total synthesis of the polyether antibiotic X-206". Journal of the American Chemical Society. 110: 2506–2526. doi:10.1021/ja00216a026.
- ↑ Baer,, E. (1942). "Oxidative Cleavage of Cyclic α-Keto Alcohols by Means of Lead Tetraacetate". Journal of the American Chemical Society. 64: 1416–1421. doi:10.1021/ja01258a049.
- ↑ Arseniyadis, Yashunsky, de Freitas, Dorado, Potier, Toupet, S.; D. V.; R. P.; M. M.; P.; L. (1996). "Left and right-half taxoid building blocks from (S)-(+)-Hajos-Parrish ketone". Tetrahedron. 52: 12443–12458.
- ↑ Unaleroglu, Aviyente, Arseniyadis, C.; V.; S. (2002). "Lead Tetraacetate Mediated One-Pot Multistage Transformations: Theoretical Studies on the Diverging Behavior in the Hajos−Parrish and Wieland−Miescher Series". The Journal of Organic Chemistry. 67: 2447–2452.
- ↑ Alvarez-Manzanedaa, Chahbouna, Alvareza, Alvarez-Manzanedab, Muñoza, Jiméneza, Bouanoua, E.; R.; E.; R.; P. E.; F.; H. (2011). "Lead(IV) acetate oxidative ring-opening of 2,3-epoxy primary alcohols: a new entry to optically active α-hydroxy carbonyl compounds". Tetrahedron Letters. 52: 4017-4020.
- ↑ Perlin, A. S. (1959). "Action of Lead Tetraacetate on the Sugars". Advances in Carbohydrate Chemistry. 14: 9-61.
- ↑ Biju, Rao,, P. J.; G. S. R. S. (1999). "Aromatics to polyquinanes: A general method for the construction of tricyclo[6.3.0.04,8]-and tricyclo[6.3.0.02,6]undecanes". Tetrahedron Letters. 40: 9379–9382.
- ↑ Moricz, Gassmann, Bienz, Hesse, A.; E.; S.; M. (1995). "Synthesis of (±)-Pyrenolide B". Helvetica Chimica Acta. 78: 663–669.