Malaprade reaction
In organic chemistry, the Malaprade reaction or Malaprade oxidation is a glycol cleavage reaction in which a vicinal diol is oxidized by periodic acid or a periodate salt to give the corresponding carbonyl functional groups.[1] The reaction was first reported by Léon Malaprade[2] in 1934 and also works with beta-aminoalcohols.[3]
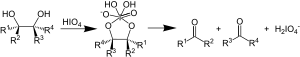 Malaprade reaction
Malaprade reaction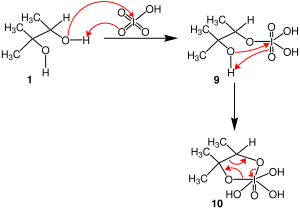 Malaprade reaction mechanism
Malaprade reaction mechanism
References
- ↑ "406. Malaprade Reaction (Malaprade Oxidation)". Comprehensive Organic Name Reactions and Reagents. Wiley. 2010. pp. 1807–1810. doi:10.1002/9780470638859.conrr406.
- ↑ L. Malaprade, Bull. Soc. Chim. Fr. 3, 1, 833 1934;
- ↑ Nicolet, Ben H.; Shinn, Leo A. (1939). "The Action of Periodic Acid on α-Amino Alcohols". J. Am. Chem. Soc. 61 (6): 1615. doi:10.1021/ja01875a521.
See also
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.