Corrole
A corrole is an aromatic organic chemical tetrapyrrole, the structure of which is similar to the corrin ring, which is also present in cobalamin (vitamin B12). The ring consists of nineteen carbon atoms, with four nitrogen atoms in the core of the molecule. In this sense, corrole is very similar to porphyrin, which is also an organic macrocycle but has twenty carbon atoms and is found in hemoglobin and chlorophyll.
Corroles can be prepared through organic synthesis by condensation reaction of a benzaldehyde with pyrrole in a water / methanol / hydrochloric acid mixture to an open-ring bilane (or tetrapyrrane) followed by oxidation and ring closure with p-chloranil:[1]
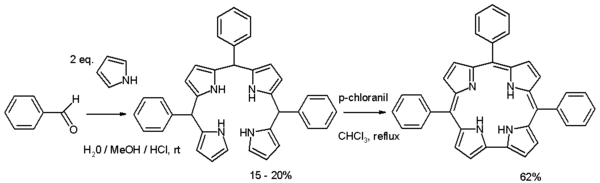
Corrole has several important differences from porphyrin. The first is that it is normally a trianionic ligand with transition metals, while porphyrin is typically a dianionic ligand. As a result, many metallocorroles are formally high-valent, even though several are noninnocent, with a corrole radical-dianion ligand.[2] The second difference from porphyrin is that, because the ring of corrole is smaller, there is slightly less space in the middle of it, which results in many metals sitting slightly out of the plane created by the four nitrogen atoms in the ring. See "Porphyrins and similar compounds" in Conjugated systems for more about these side by side images of porphyrin, chlorin, and corrin structures:
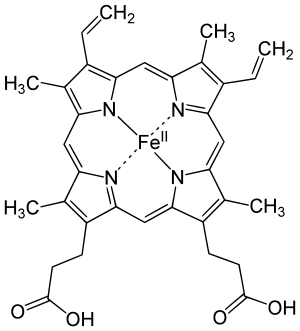 |  | 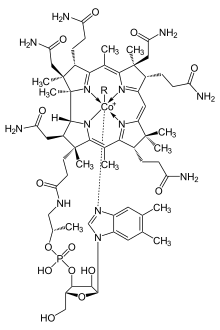 |
| Heme group of hemoglobin | The chlorin section of the chlorophyll a molecule. The green box shows a group that varies between chlorophyll types. | Cobalamin structure includes a corrin macrocycle. |
Corroles have been attached to a wide range of transition metals,[3][4][5] main group elements,[6] and more recently, lanthanides,[7] actinides.[8] and the diprotonated, neutral corrole radical.[9] Additionally, corroles and their metal complexes have been demonstrated to be useful as imaging agents in tumor detection,[10] oxygen sensing,[11] for prevention of heart disease,[12] in synthetic chemistry as oxo, imido, and nitrido transfer agents,[13] and as catalysts for the catalytic reduction of oxygen to water,[14] and hydrogen production form water under aerobic conditions.
References
- ↑ Efficient Synthesis of meso-Substituted Corroles in an H2O-MeOH Mixture Beata Koszarna and Daniel T. Gryko J. Org. Chem.; 2006; 71(10) pp 3707 - 3717. doi:10.1021/jo060007k
- ↑ Thomas, Kolle E.; Alemayehu, Abraham B.; Conradie, Jeanet; Beavers, Christine M.; Ghosh, Abhik (2012-08-21). "The Structural Chemistry of Metallocorroles: Combined X-ray Crystallography and Quantum Chemistry Studies Afford Unique Insights". Accounts of Chemical Research. 45 (8): 1203–1214. doi:10.1021/ar200292d. ISSN 0001-4842.
- ↑ Aviv-Harel, I.; Gross, Z. (2009). "Aura of Corroles". Chem. Eur. J. 15: 8382–8394. doi:10.1002/chem.200900920.
- ↑ Buckley, H. L.; Chomitz, W. A.; Koszarna, B.; Tasior, M.; Gryko, D. T.; Brothers, P. J.; Arnold, J. (2012). "Synthesis of lithium corrole and its use as a reagent for the preparation of cyclopentadienyl zirconium and titanium corrole complexes". Chem. Commun. 48: 10766–10768. doi:10.1039/c2cc35984g.
- ↑ Ghosh, Abhik (2017-02-22). "Electronic Structure of Corrole Derivatives: Insights from Molecular Structures, Spectroscopy, Electrochemistry, and Quantum Chemical Calculations". Chemical Reviews. 117 (4): 3798–3881. doi:10.1021/acs.chemrev.6b00590. ISSN 0009-2665.
- ↑ Aviv-Harel, I.; Gross, Z. (2010). "Coordination chemistry of corroles with focus on main group elements". Coord. Chem. Rev. 255: 717–736. doi:10.1016/j.ccr.2010.09.013.
- ↑ Buckley, H. L.; Anstey, M. R.; Gryko, D. T.; Arnold, J. (2013). "Lanthanide corroles: a new class of macrocyclic lanthanide complexes". Chem. Commun. 49: 3104–3106. doi:10.1039/c3cc38806a.
- ↑ Ward, A. L.; Buckley, H. L.; Lukens, W. W.; Arnold, J. (2013). "Synthesis and Characterization of Thorium(IV) and Uranium(IV) Corrole Complexes". J. Am. Chem. Soc. 135: 13965–13971. doi:10.1021/ja407203s. PMID 24004416.
- ↑ Schweyen P, Brandhorst K, Wicht R, Wolfram B, Bröring M (2015). "The Corrole Radical". Angew. Chem. Int. Ed. 54 (28): 8213–8216. doi:10.1002/anie.201503624. PMID 26074281.
- ↑ Agadjanian, H.; Ma, J.; Rentsendorj, A.; Valluripalli, V.; Youn, J.; Mahammed, A.; Farkas, D. L.; Gray, H. B.; Gross, Z.; Medina-Kauwe, L. K. (2009). "Tumor detection and elimination by a targeted gallium corrole". PNAS. 106: 6105–6110. doi:10.1073/pnas.0901531106. PMC 2669340.
- ↑ Borisov, Sergey M.; Alemayehu, Abraham; Ghosh, Abhik. "Osmium-nitrido corroles as NIR indicators for oxygen sensors and triplet sensitizers for organic upconversion and singlet oxygen generation". Journal of Materials Chemistry C. 4: 5822–5828. doi:10.1039/C6TC01126H. ISSN 2050-7534.
- ↑ Haber, Adi; Ali, A. A.-Y.; Aviram, M.; Gross, Z. (2013). "Allosteric inhibitors of HMG-CoA reductase, the key enzyme involved in cholesterol biosynthesis". Chem. Commun. 49: 10917–10919. doi:10.1039/c3cc44740e.
- ↑ Palmer, J. H. (2012). "Transition Metal Corrole Coordination Chemistry". Struct. Bond. 142: 49–90. doi:10.1007/430_2011_52.
- ↑ Dogutan, D. K.; Stoian, S. A.; McGuire, R.; Schwalbe, M.; Teets, T. S.; Nocera, D. G. (2011). "Hangman Corroles: Efficient Synthesis and Oxygen Reaction Chemistry". J. Am. Chem. Soc. 133: 131–140. doi:10.1021/ja108904s. PMID 21142043.