Contralateral brain
The contralateral organization of the forebrain (Latin: contra ‚against‘; latus ‚side‘, lateral ‚sided‘) is the property that the hemispheres of the cerebrum and the thalamus represent mainly the contralateral side of the body. Consequently, the left side of the forebrain mostly represents the right side of the body and the right side of the brain represents mostly the left side of the body. The contralateral organization involves both, executive and sensory functions (e.g., a left-sided brain lesion may cause a right-sided hemiplegia). The contralateral organization is present in all vertebrates but in no invertebrate. According to current theories, the forebrain is twisted about the long axis of the body, so that not only the left and right sides, but also dorsal and ventral sides are interchanged (see below).
Anatomy
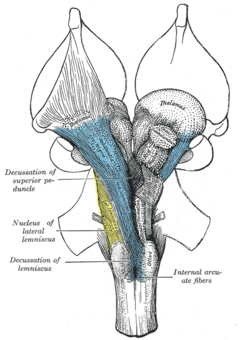
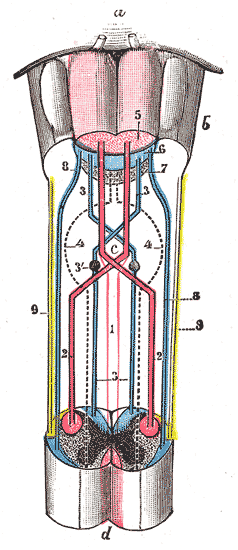
Anatomically, the contralateral organization is manifested by major decussations (latin: the Latin notation for ten, 'deca', is an uppercase 'X') and chiasmas (after the Greek uppercase letter 'Χ', chi). A decussation denotes a crossing of bundles of axonal fibres inside the central nervous system. As a result of such decussations, the efferent connections of the cerebrum to the basal ganglia, the cerebellum and the spine are crossed, and the afferent connections from the spine, the cerebellum and the pons to the thalamus are crossed.[1] As a result, motor, somatosensory, auditory, and visual primary regions in the forebrain represent predominantly the contralateral side of the body.
Two of the cranial nerves show chiasmas: the optic tract (nerve II) which originates from the eyes and inserts on the optic tectum of the midbrain, and the trochlear nerve (nerve IV) which originates in the ventral midbrain and innervates one of the six muscles that rotate the eye (superior oblique muscle).
The contralateral organization is incomplete
Although the forebrain of all vertebrates shows a contralateral organization, this contralaterality is by no means complete. Some of these exceptions are worth to mention:
- Olfaction (smelling sense): Each olfactory lobe connects to the ipsilateral centers of the frontal cerebrum.
- In chondrichthyans (sharks and skates) the thalamus does not retrieve a branch from the optic tract but only from the contralateral optic tectum, so that the optic path decussates twice and the forebrain represents the ipsilateral eye.[2][3]
- In large brains, some functions tend to be strongly lateralized. E.g., the language regions (Broca's and Wernicke's area) are in the left side in most humans.
- Most afferent and efferent connections of the forebrain have bilateral components, especially outside the primary sensory and motor regions. As a result, a hemiplegia that is acquired at very young age can be completely compensated over time.
Theories
According to current understanding, the contralateral organization is due to an axial twist (see below). A number of alternative proposals have been published earlier,[4] the most popular of which is the visual map theory.
Visual map theory by Cajal
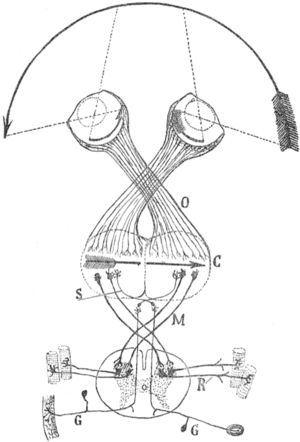
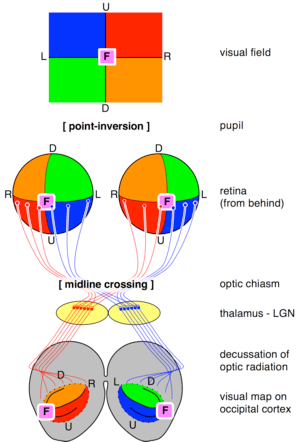
Cajal's schema of the visual map theory. O=Optic chiasm; C=Visual (and motor) cortex; M, S=Decussating pathways; R, G: Sensory nerves, motor ganglia.Transformations of the visual field toward the visual map on the primary visual cortex. U=up; D=down; L=left; R=right; F=foveaThe visual map theory was published by the famous neuroscientist and pioneer Santiago Ramón y Cajal (1898),[5] see also [6] and [4] for English summaries. According to this theory, the function of the optic chiasm is to repair the retinal field image on the visual cortex. The pupil in the vertebrates’ eyes inverts the image on the retina, so that the visual periphery projects to the medial side of the retina. By the chiasmatic crossing, the visual periphery is again on the outside, if one assumes that the retinal map is faithfully maintained throughout the optic tract.
The theory has a number of weaknesses.[7] For example, the visual tracts spiral their way from the thalamic LGN to the visual cortex. As a result, the retinal map show the visual periphery on the medial side. However, the central point of the theory was exactly to obtain a faithful visual map with the medial field projecting to the medial sides of the visual cortex.
Twist theories
Two twist theories have been proposed independently, the axial twist theory by de Marc Lussanet and Jan Osse[7] and the somatic twist theory by Marcel Kinsbourne.[8]
Axial twist theory
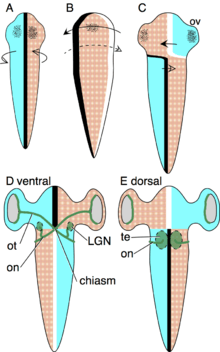
The Axial Twist Theory was designed to explain how the pattern of contralateral organization, decussations and chiasms develops, and why this pattern is evolutionary stable.[7][9] The evolutionary stability is truly remarkable, given that there are no known exceptions throughout the 500 million years of vertebrate evolution. According to the theory, the contralateral organization develops as follows. The early embryo is turned onto its left side, such that its left is turned to the yolk and its right is turned away from the yolk. This asymmetric orientation is compensated by asymmetric growth to regain superficial bilateral symmetry. The anterior head region turns to the left, as shown in the schema. The forebrain is not a superficial structure, but it is so intimately associated with superficial body structures that it turns along with the anterior head. These structures will later form the eyes, nostrils and mouth.
The body behind the head compensates the asymmetric body orientation in the opposite direction, by turning to the right (see schema). Due to these oppositely directed compensations of the anterior head and the rest of the body, the animal becomes twisted.
The Optic tract grows from the retina to the optic tectum, as dorsal and ventral are inverted in the anterior head region, the tracts grow at first to the ventral side to meet in the midline to form a chiasma. Since the opic tectum lies on the dorsal midbrain, each tract then continues dorsally to the contralateral optic tectum.
The heart and bowels are internal organs with no strong integration in external body structures, so there is no evolutionary pressure to make them turn also. Rather, these organs retain their original asymmetric orientation in the body.
Somatic twist theory
The idea of a somatic twist was inspired by the dorsoventral inversion hypothesis;[10][11] the idea was worked out by Marcel Kinsbourne.[8]
According to the dorsoventral inversion hypothesis, an ancestral deuterostome turned on its back. As a result, vertebrates have a dorsal nervous system whereas protostomes have a ventral one. According to the somatic twist hypothesis, not the entire animal turned on its back but just the ″somatic″ part, i.e., everything behind the eyes, mouth and nostrils, including the forebrain.
Comparing Inversion, Somatic twist and Axial twist
The three theories are closely related. The Somatic twist hypothesis was proposed as an improvement to the Inversion hypothesis, and thus has a much wider explanatory power than its predecessor. It not only explains the inversion of the body but additionally the contralateral forebrain. The Axial twist theory was defined independently of the other two. In addition to the inverted body and the contralateral forebrain it explains why the heart and bowels are asymmetric. Moreover, it is the only of the three that is supported by evidence from embryological growth.[9]
Evolution
A remarkable property of the contralateral organization is that it is present in every vertebrate. Even the most distant clades agnathans possess an optic chiasm.[1] and even the skull impressions of early vertebrates from the Ordovician show the presence of an optic chiasm.;[12] the idea was worked out by Kinsbourne.[8] There is molecular evidence for the inversion hypothesis in almost all groups of deuterostomes. It is not known, however, what exactly was the selective pressure that caused the inversion. Twisting and asymmetric development are well known from other deuterostomians such as Hemichordata, Echinodermata, Cephalochordata and Tunicata. Turning in the side or upside-down also occurs frequently in these clades.
Developmental malformations
In holoprosencephaly the hemispheres of the cerebrum or part of it are not aligned on the left and right side but on the frontal and occipital side of the skull, and usually remains very small. According to the axial twist hypothesis, this represents an extreme case of Yakovlevian torque,[13] and may occur when the cerebrum does not turn during early embryology.
Cephalopagus or janiceps twins are conjoined twins which are born with two faces, one on either side of the head. These twins have two brains and two spinal chords, but these are located on the left and the right side of the body.[14] According to the axial twist theory, the two nervous systems could not turn due to the complex configuration of the body and therefore remained on either side.
See also
References
- 1 2 Nieuwenhuys, R.; Donkelaar, H.J.; Nicholson, C.; Smeets, W.J.A.J.; Wicht, H. (1998). The central nervous system of vertebrates. New York: Springer.
- ↑ Luiten, P.G.M. (1981). "Two visual pathways to the telencephalon in the nurse shark (Ginglymostoma cirratum). I. retinal projections". J. Comp. Neurol. 96 (4): 531–538. doi:10.1002/cne.901960402. PMID 7204669.
- ↑ Luiten, P.G.M. (1981). "Two visual pathways to the telencephalon in the nurse shark (Ginglymostoma cirratum). II. ascending thalamo-telencephalic connections". J. Comp. Neurol. 96 (4): 539–548. doi:10.1002/cne.901960403. PMID 7204670.
- 1 2 Vulliemoz, S.; Raineteau, O.; Jabaudon, D. (2005). "Reaching beyond the midline: why are human brains cross wired?". The Lancet Neurology. 4 (2): 87–99. doi:10.1016/S1474-4422(05)00990-7. PMID 15664541.
- ↑ Ramón y Cajal, Santiago (1898). "Estructura del quiasma óptico y teoría general de los entrecruzamientos de las vías nerviosas. (Structure of the Chiasma opticum and general theory of the crossing of nerve tracks)" [Die Structur des Chiasma opticum nebst einer allgemeine Theorie der Kreuzung der Nervenbahnen (German, 1899, Verlag Joh. A. Barth)]. Rev. Trim. Micrográfica (in Spanish). 3: 15–65.
- ↑ Llinás, R.R. (2003). "The contribution of Santiago Ramón y Cajal to functional neuroscience". Nat. Rev. Neurosci. 4 (1): 77–80. doi:10.1038/nrn1011. PMID 12511864.
- 1 2 3 de Lussanet, M.H.E.; Osse, J.W.M. (2012). "An ancestral axial twist explains the contralateral forebain and the optic chiasm in vertebrates" (PDF). Animal Biology. 62: 193–216. arXiv:1003.1872. doi:10.1163/157075611X617102.
- 1 2 3 Kinsbourne, M. (2013). "Somatic twist: a model for the evolution of decussation". Neuropsychology. 27 (5): 511–515. doi:10.1037/a0033662. PMID 24040928.
- 1 2 de Lussanet, M.H.E.; Osse, J.W.M. (2015). "Decussation as an axial twist: A comment on Kinsbourne (2013)" (PDF). Neuropsychology. 29 (5): 713–714. doi:10.1037/neu0000163. PMID 25528610.
- ↑ Dixon, A. Francis (1907). "Why are the great motor and sensory tracts of the central nervous system crossed?". The Dublin Journal of Medical Science. 124 (1): 1–4. doi:10.1007/BF02972358.
- ↑ Kinsbourne, M. (1978). Asymmetrical function of the brain. Cambridge: Cambridge University Press. p. 5.
- ↑ Janvier, P. (1996). Early vertebrates. New York: Clarendon Press, Oxford University Press. ISBN 0198540477.
- ↑ Toga, A.W.; Thompson, P.M. (2003). "Mapping brain asymmetry". Nat. Rev. Neurosci. 4 (1): 37–48. doi:10.1038/nrn1009.
- ↑ Viggiano, D.; Pirolo, L. (2002). "Testing the model of optic chiasm formation in human beings". Brain Res. Bull. 59 (2): 111–115. doi:10.1016/S0361-9230(02)00846-8. PMID 12379441.